Analysis of Lenvatinib's Efficacy against Intermediate-Stage Unresectable Hepatocellular Carcinoma
- PMID: 36291850
- PMCID: PMC9600304
- DOI: 10.3390/cancers14205066
Analysis of Lenvatinib's Efficacy against Intermediate-Stage Unresectable Hepatocellular Carcinoma
Abstract
Transarterial chemoembolization (TACE) has been the standard treatment for intermediate-stage, unresectable hepatocellular carcinoma (u-HCC). However, with recent advances in systemic therapy and the emergence of the concept of TACE-refractory or -unsuitable, the effectiveness of systemic therapy, as well as TACE, has been demonstrated for patients judged to be TACE-refractory or -unsuitable. In this study, the efficacy of lenvatinib and its combination with TACE after lenvatinib was investigated in 140 patients with intermediate-stage u-HCC treated with lenvatinib mainly because of being judged to be TACE-refractory or -unsuitable. Median overall survival (OS) and progression-free survival (PFS) were 24.4 and 9.0 months, respectively, indicating a good response rate. In multivariate analysis, modified albumin-bilirubin (mALBI) grade and up to seven criteria were identified as independent factors for OS, and mALBI grade and tumor morphology were identified as independent factors for PFS. While 95% of all patients were TACE-refractory or -unsuitable, the further prognosis was prolonged by the combination with TACE after lenvatinib initiation. These findings suggest that systemic therapy should be considered for intermediate-stage u-HCC, even in patients judged to be TACE-refractory or -unsuitable. The use of TACE after the start of systemic therapy may further improve prognosis.
Keywords: LEN-TACE sequential therapy; hepatocellular carcinoma; intermediate stage; lenvatinib; modified Response Evaluation Criteria in Solid Tumors (mRECIST); overall survival; radiological response.
Conflict of interest statement
Michio Imamura has received research funding from Bristol-Myers Squibb and AbbVie. Hiroshi Aikata has received honoraria from Eisai and Bayer. All other authors declare no conflicts of interest.
Figures
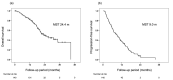
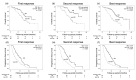

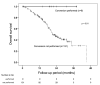
References
-
- Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. - DOI - PubMed
-
- Bruix J., Qin S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Breder V., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

