CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer
- PMID: 36291875
- PMCID: PMC9600181
- DOI: 10.3390/cancers14205093
CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer
Abstract
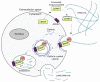
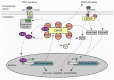
Originally discovered as a hypothetical protein with unknown function, CEMIP (cell migration-inducing and hyaluronan-binding protein) has been implicated in the pathogenesis of numerous diseases, including deafness, arthritis, atherosclerosis, idiopathic pulmonary fibrosis, and cancer. Although a comprehensive definition of its molecular functions is still in progress, major functions ascribed to CEMIP include the depolymerization of the extracellular matrix component hyaluronic acid (HA) and the regulation of a number of signaling pathways. CEMIP is a promising biomarker for colorectal cancer. Its expression is associated with poor prognosis for patients suffering from colorectal and other types of cancer and functionally contributes to tumor progression and metastasis. Here, we review our current understanding of how CEMIP is able to foster the process of tumor growth and metastasis, focusing particularly on colorectal cancer. Studies in cancer cells suggest that CEMIP exerts its pro-tumorigenic and pro-metastatic activities through stimulating migration and invasion, suppressing cell death and promoting survival, degrading HA, regulating pro-metastatic signaling pathways, inducing the epithelial-mesenchymal transition (EMT) program, and contributing to the metabolic reprogramming and pre-metastatic conditioning of future metastatic microenvironments. There is also increasing evidence indicating that CEMIP may be expressed in cells within the tumor microenvironment that promote tumorigenesis and metastasis formation, although this remains in an early stage of investigation. CEMIP expression and activity can be therapeutically targeted at a number of levels, and preliminary findings in animal models show encouraging results in terms of reduced tumor growth and metastasis, as well as combating therapy resistance. Taken together, CEMIP represents an exciting new player in the progression of colorectal and other types of cancer that holds promise as a therapeutic target and biomarker.
Keywords: CEMIP; EMT; KIAA1199; hyaluronic acid; hyaluronidase; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The crucial role of CEMIP in cancer metastasis: Mechanistic insights and clinical implications.FASEB J. 2025 Jan 15;39(1):e70284. doi: 10.1096/fj.202402522R. FASEB J. 2025. PMID: 39758005 Free PMC article. Review.
-
Research on the biological mechanism and potential application of CEMIP.Front Immunol. 2023 Aug 18;14:1222425. doi: 10.3389/fimmu.2023.1222425. eCollection 2023. Front Immunol. 2023. PMID: 37662915 Free PMC article. Review.
-
AMPK/GSK3β/β-catenin cascade-triggered overexpression of CEMIP promotes migration and invasion in anoikis-resistant prostate cancer cells by enhancing metabolic reprogramming.FASEB J. 2018 Jul;32(7):3924-3935. doi: 10.1096/fj.201701078R. Epub 2018 Mar 5. FASEB J. 2018. PMID: 29505302
-
Silencing of CEMIP suppresses Wnt/β-catenin/Snail signaling transduction and inhibits EMT program of colorectal cancer cells.Acta Histochem. 2018 Jan;120(1):56-63. doi: 10.1016/j.acthis.2017.11.002. Epub 2017 Nov 22. Acta Histochem. 2018. PMID: 29173982
-
CEMIP-mediated hyaluronan metabolism facilitates SCLC metastasis by activating TLR2/c-Src/ERK1/2 axis.Biochim Biophys Acta Mol Cell Res. 2023 Jun;1870(5):119451. doi: 10.1016/j.bbamcr.2023.119451. Epub 2023 Mar 15. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36931608
Cited by
-
TOP2A inhibition and its cellular effects related to cell cycle checkpoint adaptation pathway.Sci Rep. 2025 Jan 30;15(1):3831. doi: 10.1038/s41598-025-87895-8. Sci Rep. 2025. PMID: 39885205 Free PMC article.
-
The role of hyaluronan in renal cell carcinoma.Front Immunol. 2023 Mar 2;14:1127828. doi: 10.3389/fimmu.2023.1127828. eCollection 2023. Front Immunol. 2023. PMID: 36936902 Free PMC article. Review.
-
Targeted Analysis of the Size Distribution of Heavy Chain-Modified Hyaluronan with Solid-State Nanopores.Anal Chem. 2024 Jan 30;96(4):1606-1613. doi: 10.1021/acs.analchem.3c04387. Epub 2024 Jan 12. Anal Chem. 2024. PMID: 38215004 Free PMC article.
-
Editorial: Antioxidants and inflammatory immune-related diseases.Front Immunol. 2024 Aug 19;15:1476887. doi: 10.3389/fimmu.2024.1476887. eCollection 2024. Front Immunol. 2024. PMID: 39224592 Free PMC article. No abstract available.
-
Single-cell RNA Sequencing Analysis Reveals the Role of Cancerassociated Fibroblasts in Skin Melanoma.Curr Med Chem. 2024;31(42):7015-7029. doi: 10.2174/0109298673282799231211113347. Curr Med Chem. 2024. PMID: 38173195
References
-
- Yoshida H., Nagaoka A., Kusaka-Kikushima A., Tobiishi M., Kawabata K., Sayo T., Sakai S., Sugiyama Y., Enomoto H., Okada Y., et al. KIAA1199, a Deafness Gene of Unknown Function, Is a New Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization. Proc. Natl. Acad. Sci. USA. 2013;110:5612–5617. doi: 10.1073/pnas.1215432110. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

