Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a "MEMMAT-like" Metronomic Antiangiogenic Approach
- PMID: 36291912
- PMCID: PMC9601092
- DOI: 10.3390/cancers14205128
Improved Long-Term Survival of Patients with Recurrent Medulloblastoma Treated with a "MEMMAT-like" Metronomic Antiangiogenic Approach
Abstract
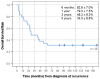
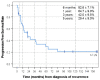
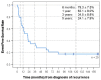
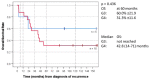
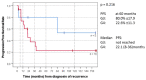
Medulloblastoma (MB) recurrence is usually incurable despite intensive therapy including high-dose chemotherapy. An evolving alternative approach to conventional chemotherapy aims at interfering with tumor angiogenesis at different levels. We report on a novel combinatorial metronomic antiangiogenic approach. The study is a retrospective observational study of 29 consecutive patients with first or multiple recurrences prospectively treated according to the MEMMAT strategy ("MEMMAT-like") before the formal protocol (MEMMAT; ClinicalTrials.gov Identifier: NCT01356290) started. The study period was 11/2006 to 06/2016. Treatment consisted of daily oral thalidomide, fenofibrate, celecoxib, and alternating 21-day cycles of low-dose oral etoposide and cyclophosphamide supplemented by IV bevacizumab and intraventricular therapy consisting of alternating etoposide and liposomal cytarabine. Median overall survival (OS) after recurrence for the whole group was 29.5 months, OS was 48.3 ± 9.3% at three years and 34.5 ± 8.8% at five years, and progression-free survival was 42.0 ± 9.5% at three years and 29.4 ± 9% at five years. As of 07/2022, 9/29 patients are alive 86 to 164 months after the recurrence that prompted the "MEMMAT-like" therapy. Treatment was primarily out-patient and generally well-tolerated. Toxicities did occur but were manageable. In conclusion, antiangiogenic therapy according to the MEMMAT strategy increased median OS of patients with recurrent MB and may lead to long-term survival. Adherence to the protocol, including intraventricular therapy, appears important.
Keywords: MEMMAT; antiangiogenic therapy; bevacizumab; intraventricular therapy; low-dose oral therapy; medulloblastoma recurrence; metronomic therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Ostrom Q.T., Price M., Ryan K., Edelson J., Neff C., Cioffi G., Waite K.A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro-Oncol. 2022;24:iii1–iii38. doi: 10.1093/neuonc/noac161. - DOI - PMC - PubMed
-
- Michalski J., Vezina G., Burger P., Gajjar A., Pollack I., Merchant T., Fitzgerald T., Booth T., Tarbell N., Shieh I., et al. Mb-109preliminary results of cog acns0331: A phase iii trial of involved field radiotherapy (ifrt) and low dose craniospinal irradiation (ld-csi) with chemotherapy in average risk medulloblastoma: A report from the children’s oncology group. Neuro-Oncol. 2016;18:iii122. doi: 10.1093/neuonc/now076.104. - DOI
-
- Tarbell N.J., Friedman H., Polkinghorn W.R., Yock T., Zhou T., Chen Z., Burger P., Barnes P., Kun L. High-Risk Medulloblastoma: A Pediatric Oncology Group Randomized Trial of Chemotherapy before or after Radiation Therapy (POG 9031) J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31:2936–2941. doi: 10.1200/JCO.2012.43.9984. - DOI - PMC - PubMed
-
- Hoff K.v., Hinkes B., Gerber N.U., Deinlein F., Mittler U., Urban C., Benesch M., Warmuth-Metz M., Soerensen N., Zwiener I., et al. Long-Term Outcome and Clinical Prognostic Factors in Children with Medulloblastoma Treated in the Prospective Randomised Multicentre Trial HIT’91. Eur. J. Cancer Oxf. Engl. 1990. 2009;45:1209–1217. doi: 10.1016/j.ejca.2009.01.015. - DOI - PubMed
-
- Johnston D.L., Keene D., Kostova M., Lafay-Cousin L., Fryer C., Scheinemann K., Carret A.-S., Fleming A., Percy V., Afzal S., et al. Survival of Children with Medulloblastoma in Canada Diagnosed between 1990 and 2009 Inclusive. J. Neurooncol. 2015;124:247–253. doi: 10.1007/s11060-015-1831-0. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

