The Vitamin D Receptor-BIM Axis Overcomes Cisplatin Resistance in Head and Neck Cancer
- PMID: 36291915
- PMCID: PMC9600548
- DOI: 10.3390/cancers14205131
The Vitamin D Receptor-BIM Axis Overcomes Cisplatin Resistance in Head and Neck Cancer
Abstract
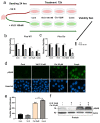
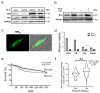
Treatment success of head and neck squamous cell carcinoma (HNSCC) is often hindered by cisplatin resistance. As inherent and acquired therapy resistance counteracts improvement in long-term survival, novel multi-targeting strategies triggering cancer cell apoptosis are urgently required. Here, we identify the vitamin D receptor (VDR) as being significantly overexpressed in tumors of HNSCC patients (n = 604; p = 0.0059), correlating with tumor differentiation (p = 0.0002), HPV status (p = 0.00026), and perineural invasion (p = 0.0087). The VDR, a member of the nuclear receptor superfamily, is activated by its ligand vitamin D (VitD) and analogs, triggering multiple cellular responses. As we found that the VDR was also upregulated in our cisplatin-resistant HNSCC models, we investigated its effect on overcoming cisplatin resistance. We discovered that VitD/cisplatin combinations synergistically killed even cisplatin-resistant cells at clinically achievable levels. Similar results were obtained for the clinically used VitD analog Maxacalcitol. Moreover, VitD/cisplatin combinations inhibited tumor cell migration by E-cadherin upregulation. Signaling pathway analyses revealed that VitD co-treatments triggered cancer cell death by increasing the expression of the pro-apoptotic BCL-2 family protein BIM. BIM's pro-apoptotic activity in HNSCC cells was confirmed by ectopic overexpression studies. Importantly, BIM expression is positively associated with HNSCC patients' (n = 539) prognosis, as high expression correlated with improved survival (p = 0.0111), improved therapy response (p = 0.0026), and remission (p = 0.004). Collectively, by identifying, for the first time, the VDR/BIM axis, we here provide a molecular rationale for the reported anti-cancer activity of VitD/analogs in combination therapies. Our data also suggest its exploitation as a potential strategy to overcome cisplatin resistance in HNSCC and other malignancies by inducing additional pro-apoptotic pathways.
Keywords: HPV; calcitriol; combination therapy; nuclear receptors; platinum-based drugs; pro-apoptotic pathways; therapy resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Siemer S., Fauth T., Scholz P., Al-Zamel Y., Khamis A., Gül D., Freudelsperger L., Wollenberg B., Becker S., Stauber R.H., et al. Profiling Cisplatin Resistance in Head and Neck Cancer: A Critical Role of the VRAC Ion Channel for Chemoresistance. Cancers. 2021;13:4831. doi: 10.3390/cancers13194831. - DOI - PMC - PubMed
-
- Gul D., Schweitzer A., Khamis A., Knauer S.K., Ding G.B., Freudelsperger L., Karampinis I., Strieth S., Hagemann J., Stauber R.H. Impact of Secretion-Active Osteoblast-Specific Factor 2 in Promoting Progression and Metastasis of Head and Neck Cancer. Cancers. 2022;14:2337. doi: 10.3390/cancers14092337. - DOI - PMC - PubMed
-
- Zhang X., Ekanayake Weeramange C., Hughes B.G.M., Vasani S., Liu Z.Y., Warkiani M.E., Hartel G., Ladwa R., Thiery J.P., Kenny L., et al. Application of circulating tumour cells to predict response to treatment in head and neck cancer. Cell. Oncol. 2022;45:543–555. doi: 10.1007/s13402-022-00681-w. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

