Multiparametric Characterization of Intracranial Gliomas Using Dynamic [18F]FET-PET and Magnetic Resonance Spectroscopy
- PMID: 36292019
- PMCID: PMC9601276
- DOI: 10.3390/diagnostics12102331
Multiparametric Characterization of Intracranial Gliomas Using Dynamic [18F]FET-PET and Magnetic Resonance Spectroscopy
Abstract
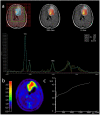
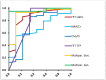
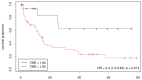
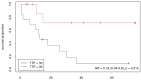
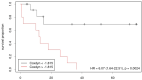
Both static and dynamic O-(2-[18F]fluoroethyl)-l-tyrosine-(FET)-PET and 1H magnetic resonance spectroscopy (MRS) are useful tools for grading and prognostication in gliomas. However, little is known about the potential of multimodal imaging comprising both procedures. We therefore acquired NAA/Cr and Cho/Cr ratios in multi-voxel MRS as well as FET-PET parameters in 67 glioma patients and determined multiparametric parameter combinations. Using receiver operating characteristics, differentiation between low-grade and high-grade glioma was possible by static FET-PET (area under the curve (AUC) 0.86, p = 0.001), time-to-peak (TTP; AUC 0.79, p = 0.049), and using the Cho/Cr ratio (AUC 0.72, p = 0.039), while the multimodal analysis led to improved discrimination with an AUC of 0.97 (p = 0.001). In order to distinguish glioblastoma from non-glioblastoma, MRS (NAA/Cr ratio, AUC 0.66, p = 0.031), and dynamic FET-PET (AUC 0.88, p = 0.001) were superior to static FET imaging. The multimodal analysis increased the accuracy with an AUC of 0.97 (p < 0.001). In the survival analysis, PET parameters, but not spectroscopy, were significantly correlated with overall survival (OS, static PET p = 0.014, TTP p = 0.012), still, the multiparametric analysis, including MRS, was also useful for the prediction of OS (p = 0.002). In conclusion, FET-PET and MRS provide complementary information to better characterize gliomas before therapy, which is particularly interesting with respect to the increasing use of hybrid PET/MRI for brain tumors.
Keywords: glioma; magnetic resonance imaging; magnetic resonance spectroscopy; multiparametric imaging; positron emission tomography.
Conflict of interest statement
J.G. and B.M. report personal fees for consultancy from Brain Lab AG, outside of the submitted work.
Figures

Similar articles
-
Magnetic resonance spectroscopy for enhanced multiparametric MRI characterization of [18F]FET PET-negative gliomas.EJNMMI Res. 2025 Apr 7;15(1):37. doi: 10.1186/s13550-025-01224-8. EJNMMI Res. 2025. PMID: 40195261 Free PMC article.
-
Multiparametric Analysis Combining DSC-MR Perfusion and [18F]FET-PET is Superior to a Single Parameter Approach for Differentiation of Progressive Glioma from Radiation Necrosis.Clin Neuroradiol. 2024 Jun;34(2):351-360. doi: 10.1007/s00062-023-01372-1. Epub 2023 Dec 29. Clin Neuroradiol. 2024. PMID: 38157019
-
Static 18F-FET PET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status.Eur Radiol. 2021 Jun;31(6):4087-4096. doi: 10.1007/s00330-020-07470-9. Epub 2020 Nov 19. Eur Radiol. 2021. PMID: 33211141
-
Recent Developments of 18F-FET PET in Neuro-oncology.Curr Med Chem. 2018;25(26):3061-3073. doi: 10.2174/0929867325666171123202644. Curr Med Chem. 2018. PMID: 29173147 Review.
-
Comparison Between 18F-Dopa and 18F-Fet PET/CT in Patients with Suspicious Recurrent High Grade Glioma: A Literature Review and Our Experience.Curr Radiopharm. 2019;12(3):220-228. doi: 10.2174/1874471012666190115124536. Curr Radiopharm. 2019. PMID: 30644351 Review.
Cited by
-
Magnetic resonance spectroscopy for enhanced multiparametric MRI characterization of [18F]FET PET-negative gliomas.EJNMMI Res. 2025 Apr 7;15(1):37. doi: 10.1186/s13550-025-01224-8. EJNMMI Res. 2025. PMID: 40195261 Free PMC article.
-
Predicting IDH Mutation Status in Low-Grade Gliomas Based on Optimal Radiomic Features Combined with Multi-Sequence Magnetic Resonance Imaging.Diagnostics (Basel). 2022 Nov 30;12(12):2995. doi: 10.3390/diagnostics12122995. Diagnostics (Basel). 2022. PMID: 36553002 Free PMC article.
-
Hybrid PET/MRI in Cerebral Glioma: Current Status and Perspectives.Cancers (Basel). 2023 Jul 12;15(14):3577. doi: 10.3390/cancers15143577. Cancers (Basel). 2023. PMID: 37509252 Free PMC article. Review.
References
-
- Meyerand M.E., Pipas J.M., Mamourian A., Tosteson T.D., Dunn J.F. Classification of biopsy-confirmed brain tumors using single-voxel MR spectroscopy. AJNR Am. J. Neuroradiol. 1999;20:117–123. - PubMed
-
- Pauleit D., Floeth F., Tellmann L., Hamacher K., Hautzel H., Muller H.W., Coenen H.H., Langen K.J. Comparison of O-(2-18F-fluoroethyl)-L-tyrosine PET and 3-123I-iodo-alpha-methyl-L-tyrosine SPECT in brain tumors. J. Nucl. Med. 2004;45:374–381. - PubMed
-
- Senft C., Hattingen E., Pilatus U., Franz K., Schanzer A., Lanfermann H., Seifert V., Gasser T. Diagnostic value of proton magnetic resonance spectroscopy in the noninvasive grading of solid gliomas: Comparison of maximum and mean choline values. Neurosurgery. 2009;65:908–913; discussion 913. doi: 10.1227/01.NEU.0000356982.82378.BA. - DOI - PubMed
-
- Smith E.A., Carlos R.C., Junck L.R., Tsien C.I., Elias A., Sundgren P.C. Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR Am. J. Roentgenol. 2009;192:W45–W52. doi: 10.2214/AJR.07.3934. - DOI - PubMed
-
- Usinskiene J., Ulyte A., Bjornerud A., Venius J., Katsaros V.K., Rynkeviciene R., Letautiene S., Norkus D., Suziedelis K., Rocka S., et al. Optimal differentiation of high- and low-grade glioma and metastasis: A meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology. 2016;58:339–350. doi: 10.1007/s00234-016-1642-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources

