Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma
- PMID: 36292207
- PMCID: PMC9600277
- DOI: 10.3390/diagnostics12102518
Radioembolization-Induced Changes in Hepatic [18F]FDG Metabolism in Non-Tumorous Liver Parenchyma
Abstract
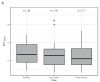
Background: [18F]FDG-PET/CT is increasingly used for response assessments after oncologic treatment. The known response criteria for [18F]FDG-PET/CT use healthy liver parenchyma as the reference standard. However, the [18F]FDG liver metabolism results may change as a result of the given therapy. The aim of this study was to assess changes in [18F]FDG liver metabolism after hepatic 90Y resin radioembolization. Methods: [18F]FDG-PET/CT scans prior to radioembolization and one and three months after radioembolization (consistent with the PERCIST comparability criteria), as well as 90Y-PET/CT scans, were analyzed using 3 cm VOIs. The FDG activity concentration and absorbed dose were measured. A linear mixed-effects logistic regression model and logistic mixed-effects model were used to assess the correlation between the FDG-activity concentration, absorbed dose, and biochemical changes. Results: The median SULVOI,liver at baseline was 1.8 (range = 1.2−2.8). The mean change in SULVOI,liver per month with an increase in time was 0.05 (95%CI 0.02−0.09) at p < 0.001. The median absorbed dose per VOI was 31.3 Gy (range = 0.1−82.3 Gy). The mean percent change in ΔSULVOI,liver for every Gy increase in the absorbed dose was −0.04 (95%CI −0.22−0.14) at p = 0.67. The SULblood and SULspleen results showed no increase. Conclusions: The [18F]FDG metabolism in the normal liver parenchyma is significantly but mildly increased after radioembolization, which can interfere with its use as a threshold for therapy response.
Keywords: FDG-PET; PERCIST; SIRT; radioembolization.
Conflict of interest statement
The authors of this manuscript declare relationships with the following companies: BTG/Boston Scientific, Terumo, and Quirem. A. Braat is a speaker for BTG/Boston Scientific and Terumo. M. Lam receives research support from Terumo, Quirem, and Boston Scientific, and is a consultant on their behalf. The department of Radiology and Nuclear Medicine of the University Medical Center Utrecht receives royalty payments from Quirem. Their radioembolization products were, however, not used in this article. The other authors of this manuscript (C. van Roekel, M.N.G.J.A. Braat) declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Figures




References
-
- Young H., Baum R., Cremerius U., Herholz K., Hoekstra O., Lammertsma A.A., Pruim J., Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. - DOI - PubMed
-
- Paquet N., Albert A., Foidart J., Hustinx R. Within-patient variability of (18)F-FDG: Standardized uptake values in normal tissues. J. Nucl. Med. 2004;45:784–788. - PubMed
-
- Kanstrup I.L., Klausen T.L., Bojsen-Moller J., Magnusson P., Zerahn B. Variability and reproducibility of hepatic FDG uptake measured as SUV as well as tissue-to-blood background ratio using positron emission tomography in healthy humans. Clin. Physiol. Funct. Imaging. 2009;29:108–113. doi: 10.1111/j.1475-097X.2008.00846.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

