Prognostic Value of Tumour-Infiltrating Lymphocytes in an Unselected Cohort of Breast Cancer Patients
- PMID: 36292215
- PMCID: PMC9601161
- DOI: 10.3390/diagnostics12102527
Prognostic Value of Tumour-Infiltrating Lymphocytes in an Unselected Cohort of Breast Cancer Patients
Abstract
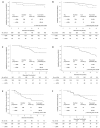
Tumour-infiltrating lymphocytes (TILs) are considered to have prognostic and predictive value for patients with early breast cancer. We examined 1166 breast cancer patients from a prospective, multicentre cohort (Prognostic Assessment in Routine Application (PiA), n = 1270, NCT01592825) following recommendations from the International TILs Working Group. TIL quantification was performed using predefined groups and as a continuous variable in 10% increments. The primary objective was the distribution of TILs in different breast cancer types. The second objective was the association with the recurrence-free interval (RFI) and overall survival (OS). Stromal infiltration with more than 60% TILs appeared in 2% of hormone receptor (HR)-positive and HER2-negative tumours, in 9.8% of HER2-positive tumours (any HR) and 19.4% of triple-negative breast cancers (TNBCs). Each 10% increment was associated with an improvement in the prognosis in HER2-positive samples (RFI, hazard ratio 0.773, 95% CI 0.587-1.017; OS, hazard ratio 0.700, 95% CI 0.523-0.937). When defining exploratory cut-offs for TILs, the use of a 30% threshold for the HR-positive and HER2-negative group, a 20% threshold for the HER2 group and a 60% threshold for the TNBC group appeared to be the most suitable. TILs bore prognostic value, especially in HER2-positive breast cancer. For clinical use, additional research on the components of immune infiltration might be reasonable.
Keywords: cohort study; early breast cancer; prognosis; tumor infiltrating lymphocytes.
Conflict of interest statement
C.T. reports support from Martin Luther University, Arbeitsgemeinschaft Gynäkologische Onkologie e.V., Sanofi-Aventis, American Diagnostica and BIOMED BMH4-98-9418, as well as honoraria from Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Gilead, Lilly, MSD, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Sanofi-Aventis, Roche, Vifor, Hexal, Mylan and Seagen. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The other authors have no relevant financial or non-financial interests to disclose.
Figures



References
-
- Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., van den Eynden G., Baehner F.L., Penault-Llorca F., et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

