Single Cell Sequencing Reveals Mechanisms of Persistent Truncus Arteriosus Formation after PDGFRα and PDGFRβ Double Knockout in Cardiac Neural Crest Cells
- PMID: 36292593
- PMCID: PMC9601305
- DOI: 10.3390/genes13101708
Single Cell Sequencing Reveals Mechanisms of Persistent Truncus Arteriosus Formation after PDGFRα and PDGFRβ Double Knockout in Cardiac Neural Crest Cells
Abstract
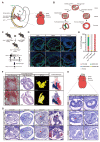
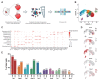
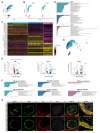
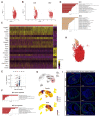
Persistent truncus arteriosus (PTA) is an uncommon and complex congenital cardiac malformation accounting for about 1.2% of all congenital heart diseases (CHDs), which is caused by a deficiency in the embryonic heart outflow tract's (OFT) septation and remodeling. PDGFRα and PDGFRβ double knockout (DKO) in cardiac neural crest cells (CNCCs) has been reported to cause PTA, but the underlying mechanisms remain unclear. Here, we constructed a PTA mouse model with PDGFRα and PDGFRβ double knockout in Pax3+ CNCCs and described the condensation failure into OFT septum of CNCC-derived cells due to disturbance of cell polarity in the DKO group. In addition, we further explored the mechanism with single-cell RNA sequencing. We found that two main cell differentiation trajectories into vascular smooth muscle cells (VSMCs) from cardiomyocytes (CMs) and mesenchymal cells (MSs), respectively, were interrupted in the DKO group. The process of CM differentiation into VSMC stagnated in a transitional CM I-like state, which contributed to the failure of OFT remodeling and muscular septum formation. On the other hand, a Penk+ transitional MS II cluster closely related to cell condensation into the OFT septum disappeared, which led to the OFT's septation absence directly. In conclusion, the disturbance of CNCC-derived cells caused by PDGFRα and PDGFRβ knockout can lead to the OFT septation disorder and the occurrence of PTA.
Keywords: cardiac outflow tract; neural crest cells; persistent truncus arteriosus; platelet-derived growth factor receptor; single-cell RNA-seq.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Conditional inactivation of Foxc1 and Foxc2 in neural crest cells leads to cardiac abnormalities.Genesis. 2020 Jul;58(7):e23364. doi: 10.1002/dvg.23364. Epub 2020 Apr 7. Genesis. 2020. PMID: 32259372 Free PMC article.
-
Over-expression of Fgf8 in cardiac neural crest cells leads to persistent truncus arteriosus.J Mol Histol. 2021 Apr;52(2):351-361. doi: 10.1007/s10735-021-09956-2. Epub 2021 Feb 5. J Mol Histol. 2021. PMID: 33547543
-
Bcar1/p130Cas is essential for ventricular development and neural crest cell remodelling of the cardiac outflow tract.Cardiovasc Res. 2022 Jun 29;118(8):1993-2005. doi: 10.1093/cvr/cvab242. Cardiovasc Res. 2022. PMID: 34270692 Free PMC article.
-
The heart of the neural crest: cardiac neural crest cells in development and regeneration.Development. 2020 Oct 15;147(20):dev188706. doi: 10.1242/dev.188706. Development. 2020. PMID: 33060096 Free PMC article. Review.
-
Cardiac outflow tract anomalies.Wiley Interdiscip Rev Dev Biol. 2013 Jul;2(4):499-530. doi: 10.1002/wdev.98. Epub 2013 Feb 19. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014420 Free PMC article. Review.
Cited by
-
Congenital scoliosis with truncus arteriosus type 1 in a preterm neonate: A case report.World J Clin Pediatr. 2025 Sep 9;14(3):106439. doi: 10.5409/wjcp.v14.i3.106439. eCollection 2025 Sep 9. World J Clin Pediatr. 2025. PMID: 40881095 Free PMC article.
References
-
- Nichols D.G., Ungerleider R.M., Spevak P.J. Critical Heart Disease in Infants and Children (Second Edition) Mosby; Philadelphia, PA, USA: 2006. pp. 995–1024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

