Influence of Parent-of-Origin on Intellectual Outcomes in the Chromosome 22q11.2 Deletion Syndrome
- PMID: 36292685
- PMCID: PMC9602066
- DOI: 10.3390/genes13101800
Influence of Parent-of-Origin on Intellectual Outcomes in the Chromosome 22q11.2 Deletion Syndrome
Abstract
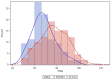
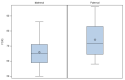
Learning and intellectual disabilities are hallmark features of 22q11.2 deletion syndrome. Data are limited, however, regarding influences on full-scale IQ (FSIQ). Here, we investigated possible 22q11.2 deletion parent-of-origin effects. In 535 individuals, we compared FSIQ (≥50), 481 with de novo and 54 with inherited 22q11.2 deletions. In the subsets with data available, we examined parent-of-origin effects on FSIQ. We used linear regression models to account for covariates. Median FSIQ was significantly higher in de novo vs. inherited deletions (77; range 50−116 vs. 67; range 50−96, p < 0.0001). Results remained significant using a regression model accounting for age at IQ testing, sex and cohort site. No significant parent-of-origin differences in FSIQ were observed for de novo deletions (n = 81, 63.0% maternal; p = 0.6882). However, median FSIQ was significantly lower in maternally than in paternally inherited familial deletions (65, range 50−86 vs. 71.5, range 58−96, respectively, p = 0.0350), with the regression model indicating an ~8 point decrement in FSIQ for this variable (p = 0.0061). FSIQ is higher on average in de novo than in inherited 22q11.2 deletions, regardless of parental origin. However, parent-of-origin appears relevant in inherited deletions. The results have potential clinical implications with further research needed to delineate possible actionable mechanisms.
Keywords: 22q; DiGeorge; FSIQ; chromosome; de novo; deletion; familial; intellect; parent-of-origin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Blagojevic C., Heung T., Theriault M., Tomita-Mitchell A., Chakraborty P., Kernohan K., Bulman D.E., Bassett A.S. Estimate of the Contemporary Live-Birth Prevalence of Recurrent 22q11.2 Deletions: A Cross-Sectional Analysis from Population-Based Newborn Screening. CMAJ Open. 2021;9:E802–E809. doi: 10.9778/cmajo.20200294. - DOI - PMC - PubMed
-
- Grati F.R., Molina Gomes D., Ferreira J.C.P.B., Dupont C., Alesi V., Gouas L., Horelli-Kuitunen N., Choy K.W., García-Herrero S., de la Vega A.G., et al. Prevalence of Recurrent Pathogenic Microdeletions and Microduplications in over 9500 Pregnancies. Prenat Diagn. 2015;35:801–809. doi: 10.1002/pd.4613. - DOI - PubMed
-
- Campbell I.M., Sheppard S.E., Crowley T.B., McGinn D.E., Bailey A., McGinn M.J., Unolt M., Homans J.F., Chen E.Y., Salmons H.I., et al. What Is New with 22q? An Update from the 22q and You Center at the Children’s Hospital of Philadelphia. Am. J. Med. Genet A. 2018;176:2058–2069. doi: 10.1002/ajmg.a.40637. - DOI - PMC - PubMed
-
- McDonald-McGinn D.M., Tonnesen M.K., Laufer-Cahana A., Finucane B., Driscoll D.A., Emanuel B.S., Zackai E.H. Phenotype of the 22q11.2 Deletion in Individuals Identified through an Affected Relative: Cast a Wide FISHing Net! Genet Med. 2001;3:23–29. doi: 10.1097/00125817-200101000-00006. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

