Advances in Novel Animal Vitamin C Biosynthesis Pathways and the Role of Prokaryote-Based Inferences to Understand Their Origin
- PMID: 36292802
- PMCID: PMC9602106
- DOI: 10.3390/genes13101917
Advances in Novel Animal Vitamin C Biosynthesis Pathways and the Role of Prokaryote-Based Inferences to Understand Their Origin
Abstract
Vitamin C (VC) is an essential nutrient required for the optimal function and development of many organisms. VC has been studied for many decades, and still today, the characterization of its functions is a dynamic scientific field, mainly because of its commercial and therapeutic applications. In this review, we discuss, in a comparative way, the increasing evidence for alternative VC synthesis pathways in insects and nematodes, and the potential of myo-inositol as a possible substrate for this metabolic process in metazoans. Methodological approaches that may be useful for the future characterization of the VC synthesis pathways of Caenorhabditis elegans and Drosophila melanogaster are here discussed. We also summarize the current distribution of the eukaryote aldonolactone oxidoreductases gene lineages, while highlighting the added value of studies on prokaryote species that are likely able to synthesize VC for both the characterization of novel VC synthesis pathways and inferences on the complex evolutionary history of such pathways. Such work may help improve the industrial production of VC.
Keywords: aldonolactone oxidoreductases; ascorbic acid; evolution; insects; nematodes; prokaryotes; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

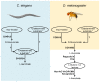
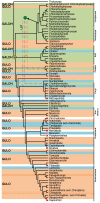
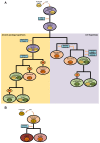
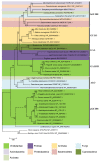
References
-
- Bauernfeind J.C. Ascorbic Acid: Chemistry, Metabolism, and Uses. American Chemical Society; Washington, DC, USA: 1982. Ascorbic Acid Technology in Agricultural, Pharmaceutical, Food, and Industrial Applications; pp. 395–497. Advances in Chemistry.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

