Role of Ion Channels in the Maintenance of Sperm Motility and Swimming Behavior in a Marine Teleost
- PMID: 36292967
- PMCID: PMC9603624
- DOI: 10.3390/ijms232012113
Role of Ion Channels in the Maintenance of Sperm Motility and Swimming Behavior in a Marine Teleost
Abstract
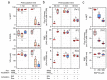
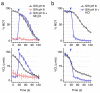
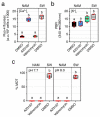
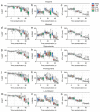
In oviparous marine fishes, the hyperosmotic induction of sperm motility in seawater (SW) is well established, however, the potential function of ion channels in the maintenance of post activated spermatozoon swimming performance remains largely unknown. Here, we investigated the influence of ion channels on the spermatozoon swimming parameters using the gilthead seabream (Sparus aurata) as a model for modern marine teleosts. Our data show that the SW-induced activation of seabream sperm motility requires three concomitant processes, the hyperosmotic shock, an ion-flux independent increase of the intracellular concentration of Ca2+ ([Ca2+]i), but not of [K+]i or [Na+]i, and the alkalization of the cytosol. The combination of all three processes is obligatory to trigger flagellar beating. However, the time-course monitoring of sperm motion kinetics and changes in the [Ca2+]i, [K+]i and [Na+]i in SW or in non-ionic activation media, showed that the post activated maintenance of spermatozoa motility is dependent on extracellular Ca2+ and K+. A meta-analysis of a seabream sperm transcriptome uncovered the expression of multiple ion channels, some of which were immunolocalized in the head and/or tail of the spermatozoon. Selective pharmacological inhibition of these ion channel families impaired the long-term motility, progressivity, and velocity of SW-activated spermatozoa. The data further revealed that some antagonists of K+-selective or Ca2+-selective channels, as well as of stretch-activated and mechanosensitive channels, altered the trajectory of spermatozoa, suggesting that these ion channels are likely involved in the control of the swimming pattern of the post activated spermatozoon. These combined findings provide new insight into the signaling pathways regulating spermatozoon activation and swimming performance in marine fishes.
Keywords: activation; ion channels; ions; motility; pH; spermatozoa; trajectory; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



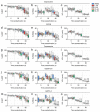
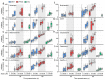

References
-
- Cosson J., Groison A., Suquet M., Fauvel C., Dreanno C., Billard R. Studying sperm motility in marine fish: An overview on the state of the art. J. Appl. Ichthyol. 2008;24:460–486. doi: 10.1111/j.1439-0426.2008.01151.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

