The Effects of High CO2 and Strigolactones on Shoot Branching and Aphid-Plant Compatibility Control in Pea
- PMID: 36293014
- PMCID: PMC9602761
- DOI: 10.3390/ijms232012160
The Effects of High CO2 and Strigolactones on Shoot Branching and Aphid-Plant Compatibility Control in Pea
Abstract
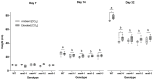
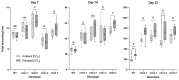
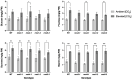
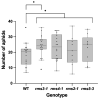
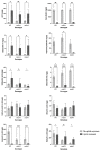
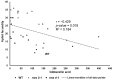
Elevated atmospheric CO2 concentrations (eCO2) regulate plant architecture and susceptibility to insects. We explored the mechanisms underpinning these responses in wild type (WT) peas and mutants defective in either strigolactone (SL) synthesis or signaling. All genotypes had increased shoot height and branching, dry weights and carbohydrate levels under eCO2, demonstrating that SLs are not required for shoot acclimation to eCO2. Since shoot levels of jasmonic acid (JA) and salicylic acid (SA) tended to be lower in SL signaling mutants than the WT under ambient conditions, we compared pea aphid performance on these lines under both CO2 conditions. Aphid fecundity was increased in the SL mutants compared to the WT under both ambient and eCO2 conditions. Aphid infestation significantly decreased levels of JA, isopentenyladenine, trans-zeatin and gibberellin A4 and increased ethylene precursor ACC, gibberellin A1, gibberellic acid (GA3) and SA accumulation in all lines. However, GA3 levels were increased less in the SL signaling mutants than the WT. These studies provide new insights into phytohormone responses in this specific aphid/host interaction and suggest that SLs and gibberellins are part of the network of phytohormones that participate in host susceptibility.
Keywords: aphids; climate change; high atmospheric carbon dioxide; phytohormones; plant architecture; strigolactones.
Conflict of interest statement
There are no conflicts of interest.
Figures

Similar articles
-
Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses.Plant Sci. 2013 Sep;210:128-40. doi: 10.1016/j.plantsci.2013.05.014. Epub 2013 Jun 2. Plant Sci. 2013. PMID: 23849120
-
Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation.Plant Sci. 2014 May;221-222:1-12. doi: 10.1016/j.plantsci.2014.01.011. Epub 2014 Jan 31. Plant Sci. 2014. PMID: 24656330
-
Elevated CO2 alters the feeding behaviour of the pea aphid by modifying the physical and chemical resistance of Medicago truncatula.Plant Cell Environ. 2014 Sep;37(9):2158-68. doi: 10.1111/pce.12306. Epub 2014 Apr 9. Plant Cell Environ. 2014. PMID: 24697655
-
Salicylic acid and jasmonic acid in elevated CO2-induced plant defense response to pathogens.J Plant Physiol. 2023 Jul;286:154019. doi: 10.1016/j.jplph.2023.154019. Epub 2023 May 20. J Plant Physiol. 2023. PMID: 37244001 Review.
-
Plant-Aphid Interactions Under Elevated CO2: Some Cues from Aphid Feeding Behavior.Front Plant Sci. 2016 Apr 13;7:502. doi: 10.3389/fpls.2016.00502. eCollection 2016. Front Plant Sci. 2016. PMID: 27148325 Free PMC article. Review.
Cited by
-
Sugar Transport and Signaling in Shoot Branching.Int J Mol Sci. 2024 Dec 9;25(23):13214. doi: 10.3390/ijms252313214. Int J Mol Sci. 2024. PMID: 39684924 Free PMC article. Review.
-
Recent Advances in Plant-Insect Interactions.Int J Mol Sci. 2023 Jul 12;24(14):11338. doi: 10.3390/ijms241411338. Int J Mol Sci. 2023. PMID: 37511097 Free PMC article.
References
-
- Zhang J., De Oliveira-Cecilato P., Takahashi Y., Schulze S., Dubeaux G., Hauser F., Azoulay-Shaemwe T., Tõldsepp K., Kollist H., Rappel W.-J., et al. Insights into the molecular mechanisms of CO2-mediated regulation of stomatal movements. Curr. Biol. 2018;28:R1356–R1363. doi: 10.1016/j.cub.2018.10.015. - DOI - PubMed
-
- Winkler A.J., Myneni R.B., Hannart A., Sitch S., Haverd V., Lombardozzi D., Arora V.K., Pongratz J., Nabel J.E.M.S., Goll D.S., et al. Slowdown of the greening trend in natural vegetation with further rise in atmospheric CO2. Biogeosciences. 2021;18:4985–5010. doi: 10.5194/bg-18-4985-2021. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

