Altered Circulating Follicular T Helper Cell Subsets and Follicular T Regulatory Cells Are Indicators of a Derailed B Cell Response in Lupus, Which Could Be Modified by Targeting IL-21R
- PMID: 36293075
- PMCID: PMC9602506
- DOI: 10.3390/ijms232012209
Altered Circulating Follicular T Helper Cell Subsets and Follicular T Regulatory Cells Are Indicators of a Derailed B Cell Response in Lupus, Which Could Be Modified by Targeting IL-21R
Abstract
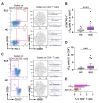
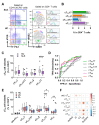
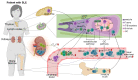
Systemic lupus erythematosus (SLE) is characterized by the breakdown of self-tolerance, the production of high-affinity pathogenic autoantibodies and derailed B cell responses, which indicates the importance of central players, such as follicular T helper (TFH) subsets and follicular T regulatory (TFR) cells, in the pathomechanism of the disease. In this study, we aimed to analyze the distribution of the circulating counterparts of these cells and their association with disease characteristics and B cell disproportions in SLE. We found that the increased percentage of activated circulating TFH (cTFH) and cTFR cells was more pronounced in cutaneous lupus; however, among cTFH subsets, the frequency of cTFH17 cells was decreased in patients with lupus nephritis. Furthermore, the decreased proportion of cTFH17 cells was associated with low complement C4 levels and high disease activity scores. We also investigated whether the blocking of the IL-21 receptor (IL-21R) with an anti-IL-21R monoclonal antibody inhibits the B cell response, since IL-21 primarily produced by TFH cells potentially promotes humoral immunity. We observed that anti-IL-21R inhibited plasmablast generation and immunoglobulin production. Our study demonstrated that, besides cTFR/cTFH imbalance, cTFH17 cells play a crucial role in SLE pathogenesis, and modulating cTFH-B cell interaction through the IL-21/IL-21R pathway may be a promising therapeutic strategy to suppress the pathological B cell response.
Keywords: B cell; chemokine receptors; follicular T helper cell; follicular T regulatory cell; interleukin-21 receptor; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- K124177/National Research, Development and Innovation Office (Hungary)
- PD121327/National Research, Development and Innovation Office (Hungary)
- ÚNKP-21-05-DE-170/New National Excellence Program of the Ministry for Innovation and Technology (National Research, Development and Innovation Office) and János Bolyai Research Scholarship (Hungarian Academy of Sciences)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

