Chitin Synthesis in Yeast: A Matter of Trafficking
- PMID: 36293107
- PMCID: PMC9603707
- DOI: 10.3390/ijms232012251
Chitin Synthesis in Yeast: A Matter of Trafficking
Abstract
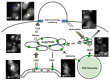
Chitin synthesis has attracted scientific interest for decades as an essential part of fungal biology and for its potential as a target for antifungal therapies. While this interest remains, three decades ago, pioneering molecular studies on chitin synthesis regulation identified the major chitin synthase in yeast, Chs3, as an authentic paradigm in the field of the intracellular trafficking of integral membrane proteins. Over the years, researchers have shown how the intracellular trafficking of Chs3 recapitulates all the steps in the intracellular trafficking of integral membrane proteins, from their synthesis in the endoplasmic reticulum to their degradation in the vacuole. This trafficking includes specific mechanisms for sorting in the trans-Golgi network, regulated endocytosis, and endosomal recycling at different levels. This review summarizes the work carried out on chitin synthesis regulation, mostly focusing on Chs3 as a molecular model to study the mechanisms involved in the control of the intracellular trafficking of proteins.
Keywords: chitin synthase; intracellular traffic; yeasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

