Optogenetic fMRI for Brain-Wide Circuit Analysis of Sensory Processing
- PMID: 36293125
- PMCID: PMC9602603
- DOI: 10.3390/ijms232012268
Optogenetic fMRI for Brain-Wide Circuit Analysis of Sensory Processing
Abstract
Sensory processing is a complex neurological process that receives, integrates, and responds to information from one's own body and environment, which is closely related to survival as well as neurological disorders. Brain-wide networks of sensory processing are difficult to investigate due to their dynamic regulation by multiple brain circuits. Optogenetics, a neuromodulation technique that uses light-sensitive proteins, can be combined with functional magnetic resonance imaging (ofMRI) to measure whole-brain activity. Since ofMRI has increasingly been used for investigating brain circuits underlying sensory processing for over a decade, we systematically reviewed recent ofMRI studies of sensory circuits and discussed the challenges of optogenetic fMRI in rodents.
Keywords: functional magnetic resonance imaging (fMRI); optogenetic fMRI (ofMRI); optogenetics; sensory processing; sensory system.
Conflict of interest statement
The authors declare no competing interest.
Figures
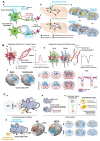
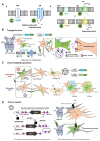
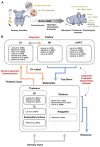
References
-
- Parham L.D., Crickmore D. Intellectual Disabilities-E-Book: Toward Inclusion. Elsevier Health Sciences; Warsaw, Poland: 2022. Expanding sensory awareness; p. 231.
-
- Greven C.U., Lionetti F., Booth C., Aron E.N., Fox E., Schendan H.E., Pluess M., Bruining H., Acevedo B., Bijttebier P., et al. Sensory Processing Sensitivity in the context of Environmental Sensitivity: A critical review and development of research agenda. Neurosci. Biobehav. Rev. 2019;98:287–305. doi: 10.1016/j.neubiorev.2019.01.009. - DOI - PubMed
-
- Galiana-Simal A., Vela-Romero M., Romero-Vela V.M., Oliver-Tercero N., García-Olmo V., Benito-Castellanos P.J., Muñoz-Martinez V., Beato-Fernandez L. Sensory processing disorder: Key points of a frequent alteration in neurodevelop-mental disorders. Cog. Med. 2020;7:1736829. doi: 10.1080/2331205X.2020.1736829. - DOI
-
- Ranford J., MacLean J., Alluri P.R., Comeau O., Godena E., LaFrance W.C., Jr., Hunt A., Stephen C.D., Perez D.L. Sensory Processing Difficulties in Functional Neurological Disorder: A Possible Predisposing Vulnerability? Psychosomatics. 2020;61:343–352. doi: 10.1016/j.psym.2020.02.003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

