Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing
- PMID: 36293130
- PMCID: PMC9602983
- DOI: 10.3390/ijms232012274
Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing
Abstract
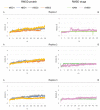
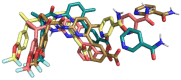
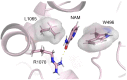
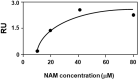
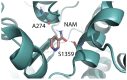
Cystic fibrosis is a hereditary disease mainly caused by the deletion of the Phe 508 (F508del) of the cystic fibrosis transmembrane conductance regulator (CFTR) protein that is thus withheld in the endoplasmic reticulum and rapidly degraded by the ubiquitin/proteasome system. Cystic fibrosis remains a potentially fatal disease, but it has become treatable as a chronic condition due to some CFTR-rescuing drugs that, when used in combination, increase in their therapeutic effect due to a synergic action. Also, dietary supplementation of natural compounds in combination with approved drugs could represent a promising strategy to further alleviate cystic fibrosis symptoms. On these bases, we screened by in silico drug repositioning 846 small synthetic or natural compounds from the AIFA database to evaluate their capacity to interact with the highly druggable lumacaftor binding site of F508del-CFTR. Among the identified hits, nicotinamide (NAM) was predicted to accommodate into the lumacaftor binding region of F508del-CFTR without competing against the drug but rather stabilizing its binding. The effective capacity of NAM to bind F508del-CFTR in a lumacaftor-uncompetitive manner was then validated experimentally by surface plasmon resonance analysis. Finally, the capacity of NAM to synergize with lumacaftor increasing its CFTR-rescuing activity was demonstrated in cell-based assays. This study suggests the possible identification of natural small molecules devoid of side effects and endowed with the capacity to synergize with drugs currently employed for the treatment of cystic fibrosis, which hopefully will increase the therapeutic efficacy with lower doses.
Keywords: F508del-CFTR; cystic fibrosis; drug repositioning; lumacaftor; molecular docking and dynamics; nicotinamide; surface plasmon resonance.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Veit G., Avramescu R.G., Chiang A.N., Houck S.A., Cai Z., Peters K.W., Hong J.S., Pollard H.B., Guggino W.B., Balch W.E., et al. From Cftr Biology toward Combinatorial Pharmacotherapy: Expanded Classification of Cystic Fibrosis Mutations. Mol. Biol. Cell. 2016;27:424–433. doi: 10.1091/mbc.e14-04-0935. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- FFC #10/2019; FFC#9/2019; FFC#10/2021/Fondazione Italiana Fibrosi Cistica
- PEDEMO20G0/Cystic Fibrosis Foundation
- 857650/EU project EOSC-Pillar
- A.R. fellowship/European research infrastructure for Biobanking and BioMolecular Resources Research Infrastructure (BBMRI)
- PON R&I PIR01_00017/CENTRO NAZIONALE DI RICERCA IN BIOINFORMATICA PER LE SCIENZE "OMICHE" CNRBIOMICS
LinkOut - more resources
Full Text Sources
Medical

