Altered Expression of AQP1 and AQP4 in Brain Barriers and Cerebrospinal Fluid May Affect Cerebral Water Balance during Chronic Hypertension
- PMID: 36293145
- PMCID: PMC9603298
- DOI: 10.3390/ijms232012277
Altered Expression of AQP1 and AQP4 in Brain Barriers and Cerebrospinal Fluid May Affect Cerebral Water Balance during Chronic Hypertension
Abstract
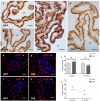
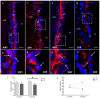
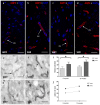
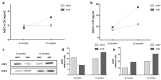
Hypertension is the leading cause of cardiovascular affection and premature death worldwide. The spontaneously hypertensive rat (SHR) is the most common animal model of hypertension, which is characterized by secondary ventricular dilation and hydrocephalus. Aquaporin (AQP) 1 and 4 are the main water channels responsible for the brain’s water balance. The present study focuses on defining the expression of AQPs through the time course of the development of spontaneous chronic hypertension. We performed immunofluorescence and ELISA to examine brain AQPs from 10 SHR, and 10 Wistar−Kyoto (WKY) rats studied at 6 and 12 months old. There was a significant decrease in AQP1 in the choroid plexus of the SHR-12-months group compared with the age-matched control (p < 0.05). In the ependyma, AQP4 was significantly decreased only in the SHR-12-months group compared with the control or SHR-6-months groups (p < 0.05). Per contra, AQP4 increased in astrocytes end-feet of 6 months and 12 months SHR rats (p < 0.05). CSF AQP detection was higher in the SHR-12-months group than in the age-matched control group. CSF findings were confirmed by Western blot. In SHR, ependymal and choroidal AQPs decreased over time, while CSF AQPs levels increased. In turn, astrocytes AQP4 increased in SHR rats. These AQP alterations may underlie hypertensive-dependent ventriculomegaly.
Keywords: SHR; aquaporins; cerebrospinal fluid; choroid plexus; ependyma; hydrocephalus; hypertension.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Expression of aquaporins 1 and 4 in the brain of spontaneously hypertensive rats.Brain Res. 2010 Apr 14;1325:155-63. doi: 10.1016/j.brainres.2010.02.023. Epub 2010 Feb 12. Brain Res. 2010. PMID: 20156423
-
Increased expression of renal aquaporin water channels in spontaneously hypertensive rats.Kidney Blood Press Res. 2006;29(1):18-23. doi: 10.1159/000092483. Epub 2006 Mar 22. Kidney Blood Press Res. 2006. PMID: 16582573
-
Aquaporin 4 changes in rat brain with severe hydrocephalus.Eur J Neurosci. 2006 Jun;23(11):2929-36. doi: 10.1111/j.1460-9568.2006.04829.x. Eur J Neurosci. 2006. PMID: 16819982
-
Aquaporin Water Channels and Hydrocephalus.Pediatr Neurosurg. 2017;52(6):409-416. doi: 10.1159/000452168. Epub 2016 Dec 16. Pediatr Neurosurg. 2017. PMID: 27978530 Free PMC article. Review.
-
Hydrocephalus and aquaporins: lessons learned from the bench.Childs Nerv Syst. 2011 Jan;27(1):27-33. doi: 10.1007/s00381-010-1227-6. Epub 2010 Jul 13. Childs Nerv Syst. 2011. PMID: 20625739 Review.
Cited by
-
Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness.Life (Basel). 2024 Dec 20;14(12):1688. doi: 10.3390/life14121688. Life (Basel). 2024. PMID: 39768394 Free PMC article. Review.
-
Aging aggravates cognitive dysfunction in spontaneously hypertensive rats by inducing cerebral microvascular endothelial dysfunction.PLoS One. 2025 Mar 13;20(3):e0316383. doi: 10.1371/journal.pone.0316383. eCollection 2025. PLoS One. 2025. PMID: 40080509 Free PMC article.
-
Blood-brain barrier pathology in cerebral small vessel disease.Neural Regen Res. 2024 Jun 1;19(6):1233-1240. doi: 10.4103/1673-5374.385864. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 37905869 Free PMC article.
-
Special Issue "Aquaporins in Brain Disease".Int J Mol Sci. 2024 Mar 20;25(6):3513. doi: 10.3390/ijms25063513. Int J Mol Sci. 2024. PMID: 38542489 Free PMC article.
-
Neural stem cell changes and spatial distribution of AQP4 expression in a fetal goat model of obstructive hydrocephalus.IBRO Neurosci Rep. 2025 Apr 5;18:627-637. doi: 10.1016/j.ibneur.2025.03.013. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40584426 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

