Chi-Circ_0006511 Positively Regulates the Differentiation of Goat Intramuscular Adipocytes via Novel-miR-87/CD36 Axis
- PMID: 36293149
- PMCID: PMC9603556
- DOI: 10.3390/ijms232012295
Chi-Circ_0006511 Positively Regulates the Differentiation of Goat Intramuscular Adipocytes via Novel-miR-87/CD36 Axis
Abstract
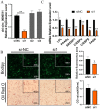
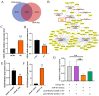
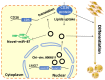
Goats are an important livestock and goat meat is essential to local people. The intramuscular fat (IMF) content has a great influence on the quality of goat meat. The intramuscular preadipocytes differentiation is closely related to the IMF deposition; however, its potential regulatory mechanisms remain unclear. CircRNAs were revealed to be involved in multiple biological progressions. In this study, we took primary goat intramuscular preadipocyte (GIMPA) as the study model to verify the function and mechanism of chi-circ_0006511, which was abundant and up-regulated in mature adipocytes (GIMA). The results showed that the expression level of chi-circ_0006511 gradually increased in the early stage of GIMPA differentiation, and chi-circ_0006511 was confirmed to promote GIMPA lipid droplets aggregation and up-regulate the adipogenic differentiation determinants, further promoting GIMPA differentiation. Mechanistically, chi-circ_0006511 exerts its function by sponging novel-miR-87, thereby regulating the expression of CD36. The results from this study provided novel significant information to better understand the molecular regulatory mechanism of intramuscular preadipocytes differentiation, thereby providing a new reference for the intramuscular fat adipogenesis in goats.
Keywords: CD36; adipocyte differentiation; circRNA; goat; novel-miR-87.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 ” indicates Divergent primer, “
” indicates Divergent primer, “ ” indicates Convergent primer.
” indicates Convergent primer.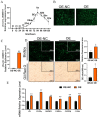



References
-
- Tefiel H., Ata N., Chahbar M., Benyarou M., Fantazi K., Yilmaz O., Cemal I., Karaca O., Boudouma D., Gaouar S.B.S. Genetic characterization of four Algerian goat breeds assessed by microsatellite markers. Small Rumin. Res. 2018;160:65–71. doi: 10.1016/j.smallrumres.2018.01.021. - DOI
-
- Stanišić N., Žujović M., Tomić Z., Maksimović N., Bijelić Z., Ivanović S., Memiši N. The Effects of Crossing Balkan and Saanen Goat Breeds on Carcass Traits and Certain Quality Parameters of Kid Meat. Ann. Anim. Sci. 2012;12:53–62. doi: 10.2478/v10220-012-0004-8. - DOI
-
- Silva Mariniello T., Oliveira Lopes R., Barbosa Pires L., Neto G., Froes A., Bagaldo A.R., Lanna D.P.D., Alves de Silva M.C., Brito de Jesus I. Preliminary Study on Meat Quality of Goats Fed Levels of Licury Oil in the Diet. Asian-Australas. J. Anim. Sci. 2011;24:1112–1119. doi: 10.5713/ajas.2011.11053. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

