A Novel Antibody-Drug Conjugate Targeting Nectin-2 Suppresses Ovarian Cancer Progression in Mouse Xenograft Models
- PMID: 36293219
- PMCID: PMC9604294
- DOI: 10.3390/ijms232012358
A Novel Antibody-Drug Conjugate Targeting Nectin-2 Suppresses Ovarian Cancer Progression in Mouse Xenograft Models
Abstract
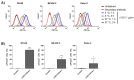
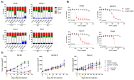
Ovarian cancer is the fifth leading cause of cancer, followed by front line is mostly platinum agents and PARP inhibitors, and very limited option in later lines. Therefore, there is a need for alternative therapeutic options. Nectin-2, which is overexpressed in ovarian cancer, is a known immune checkpoint that deregulates immune cell function. In this study, we generated a novel anti-nectin-2 antibody (chimeric 12G1, c12G1), and further characterized it using epitope mapping, enzyme-linked immunosorbent assay, surface plasmon resonance, fluorescence-activated cell sorting, and internalization assays. The c12G1 antibody specifically bound to the C2 domain of human nectin-2 with high affinity (KD = 2.90 × 10-10 M), but not to mouse nectin-2. We then generated an antibody-drug conjugate comprising the c12G1 antibody conjugated to DM1 and investigated its cytotoxic effects against cancer cells in vitro and in vivo. c12G1-DM1 induced cell cycle arrest at the mitotic phase in nectin-2-positive ovarian cancer cells, but not in nectin-2-negative cancer cells. c12G1-DM1 induced ~100-fold cytotoxicity in ovarian cancer cells, with an IC50 in the range of 0.1 nM~7.4 nM, compared to normal IgG-DM1. In addition, c12G1-DM1 showed ~91% tumor growth inhibition in mouse xenograft models transplanted with OV-90 cells. These results suggest that c12G1-DM1 could be used as a potential therapeutic agent against nectin-2-positive ovarian cancers.
Keywords: antibody-drug conjugate; chimeric antibody; nectin-2; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Meinhold-Heerlein I., Fotopoulou C., Harter P., Kurzeder C., Mustea A., Wimberger P., Hauptmann S., Sehouli J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016;293:695–700. doi: 10.1007/s00404-016-4035-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

