The Antimicrobial Peptide 1018-K6 Interacts Distinctly with Eukaryotic and Bacterial Membranes, the Basis of Its Specificity and Bactericidal Activity
- PMID: 36293249
- PMCID: PMC9603936
- DOI: 10.3390/ijms232012392
The Antimicrobial Peptide 1018-K6 Interacts Distinctly with Eukaryotic and Bacterial Membranes, the Basis of Its Specificity and Bactericidal Activity
Abstract
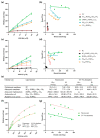
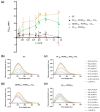
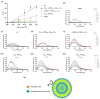
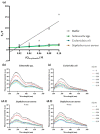
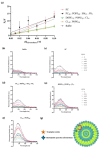
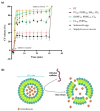
Since penicillin was discovered, antibiotics have been critical in the fight against infections. However, antibiotic misuse has led to drug resistance, which now constitutes a serious health problem. In this context, antimicrobial peptides (AMPs) constitute a natural group of short proteins, varying in structure and length, that act against certain types of bacterial pathogens. The antimicrobial peptide 1018-K6 (VRLIVKVRIWRR- NH2) has significant bactericidal and antibiofilm activity against Listeria monocytogenes isolates, and against different strains and serotypes of Salmonella. Here, the mechanism of action of 1018-K6 was explored further to understand the peptide-membrane interactions relevant to its activity, and to define their determinants. We combined studies with model synthetic membranes (liposomes) and model biological membranes, assessing the absorption maximum and the quenching of 1018-K6 fluorescence in aqueous and lipid environments, the self-quenching of carboxyfluorescein, as well as performing lipid sedimentation assays. The data obtained reflect the differential interactions of the 1018-K6 peptide with eukaryotic and prokaryotic membranes, and the specific interactions and mechanisms of action in the three prokaryotic species studied: Salmonella Typhimurium2GN, Escherichia coli3GN, and Staphylococcus aureus3GP. The AMP 1018-K6 is a candidate to prevent (food preservation) or treat (antibiotic use) infections caused by certain pathogenic bacteria, especially some that are resistant to current antibiotics.
Keywords: Salmonella spp.; Staphylococcus spp.; antimicrobial peptide; food preservation; membrane lipid therapy (melitherapy).
Conflict of interest statement
The authors have no conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Functional interplay between short antimicrobial peptides and model lipid membranes.Bioorg Chem. 2024 Dec;153:107939. doi: 10.1016/j.bioorg.2024.107939. Epub 2024 Nov 3. Bioorg Chem. 2024. PMID: 39520786
-
Synthetic peptides that form nanostructured micelles have potent antibiotic and antibiofilm activity against polymicrobial infections.Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2219679120. doi: 10.1073/pnas.2219679120. Epub 2023 Jan 17. Proc Natl Acad Sci U S A. 2023. PMID: 36649429 Free PMC article.
-
Bactericidal and antibiofilm activity of bactenecin-derivative peptides against the food-pathogen Listeria monocytogenes: New perspectives for food processing industry.Int J Food Microbiol. 2018 Aug 20;279:33-42. doi: 10.1016/j.ijfoodmicro.2018.04.039. Epub 2018 Apr 27. Int J Food Microbiol. 2018. PMID: 29727856
-
Rescuing humanity by antimicrobial peptides against colistin-resistant bacteria.Appl Microbiol Biotechnol. 2022 Jun;106(11):3879-3893. doi: 10.1007/s00253-022-11940-z. Epub 2022 May 23. Appl Microbiol Biotechnol. 2022. PMID: 35604438 Free PMC article. Review.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
Cited by
-
High-Yield Preparation of American Oyster Defensin (AOD) via a Small and Acidic Fusion Tag and Its Functional Characterization.Mar Drugs. 2023 Dec 20;22(1):8. doi: 10.3390/md22010008. Mar Drugs. 2023. PMID: 38276646 Free PMC article.
-
The Designed Pore-Forming Antimicrobial Peptide C14R Combines Excellent Activity against the Major Opportunistic Human Pathogen Pseudomonas aeruginosa with Low Cytotoxicity.Pharmaceuticals (Basel). 2024 Jan 9;17(1):83. doi: 10.3390/ph17010083. Pharmaceuticals (Basel). 2024. PMID: 38256916 Free PMC article.
-
Membrane-Peptide Interactions: From Basics to Current Applications 2.0.Int J Mol Sci. 2023 Apr 13;24(8):7202. doi: 10.3390/ijms24087202. Int J Mol Sci. 2023. PMID: 37108363 Free PMC article.
-
The limits of prediction: Why intrinsically disordered regions challenge our understanding of antimicrobial peptides.Comput Struct Biotechnol J. 2024 Feb 12;23:972-981. doi: 10.1016/j.csbj.2024.02.008. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38404711 Free PMC article.
-
ERK1/2-CEBPB Axis-Regulated hBD1 Enhances Anti-Tuberculosis Capacity in Alveolar Type II Epithelial Cells.Int J Mol Sci. 2024 Feb 18;25(4):2408. doi: 10.3390/ijms25042408. Int J Mol Sci. 2024. PMID: 38397085 Free PMC article.
References
-
- Fleming A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. Influenzæ. Br. J. Exp. Pathol. 1929;10:226–236. doi: 10.1093/clinids/2.1.129. - DOI
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Rev. Antimicrob. Resist. 2016;1:1–84.
-
- World Health Organization . The Evolving Threat of Antimicrobial Resistance: Options for Action. World Health Organization; Cham, Switzerland: 2012.
-
- Escribá P.V., Busquets X., Inokuchi J.I., Balogh G., Török Z., Horváth I., Harwood J.L., Vígh L. Membrane Lipid Therapy: Modulation of the Cell Membrane Composition and Structure as a Molecular Base for Drug Discovery and New Disease Treatment. Prog. Lipid Res. 2015;59:38–53. doi: 10.1016/j.plipres.2015.04.003. - DOI - PubMed
-
- Verkleij A.J., Zwaal R.F., Roelofsen B., Comfurius P., Kastelijn D., Deenen L.L.M. The Asymmetric Distribution of Phospholipids in the Human Red Cell Membrane. A Combined Study Using Phospholipases and Freeze-Etch Electron Microscopy. Biochim. Biophys. Acta. 1973;323:178–193. doi: 10.1016/0005-2736(73)90143-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- "Packaging Bioattivi e bIOsanitizzanti: Sviluppo di stratEgie iNnovaTIve ed ecososteNibili pEr L'Industria Alimentare- (BIO-SENTINEL) project, grant number F/200092/01-03/X45/Ministry of Economic Development
- Fondo per la Crescita Sostenibile- Sportello "Agrifood" PON I&C 2014-2020; Regione Campa-nia-"Sviluppo di una tecnologia Intelli- gente contro spoilage ed agenti patogeni: dal peptide an-timicrobico ad un PACKaging innovativo nella filiera ittica del Medi/Ministry of Economic Development
- Packaging innovativi a base di pEptidi antimicRobici per la SIcurezza Alimentare" (PERSIA) project, grant number POR FESR CAMPANIA 2014/2020 n. B63D18000530007/Regione Campania
- MCIN/AEI/10.13039/501100011033 (RTC2019-007399-1)/Ministerio de Ciencia e innovación
- Grant ES01/TCAI/24_2018/Govern de les Illes Balears
LinkOut - more resources
Full Text Sources
Miscellaneous

