The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding
- PMID: 36293357
- PMCID: PMC9604345
- DOI: 10.3390/ijms232012501
The Third Extracellular Loop of Mammalian Odorant Receptors Is Involved in Ligand Binding
Abstract
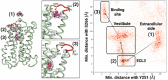
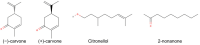
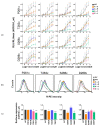
Mammals recognize chemicals in the air via G protein-coupled odorant receptors (ORs). In addition to their orthosteric binding site, other segments of these receptors modulate ligand recognition. Focusing on human hOR1A1, which is considered prototypical of class II ORs, we used a combination of molecular modeling, site-directed mutagenesis, and in vitro functional assays. We showed that the third extracellular loop of ORs (ECL3) contributes to ligand recognition and receptor activation. Indeed, site-directed mutations in ECL3 showed differential effects on the potency and efficacy of both carvones, citronellol, and 2-nonanone.
Keywords: ECL3; functional assays; ligand selectivity; molecular modeling; odorant receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Exchanging ligand-binding specificity between a pair of mouse olfactory receptor paralogs reveals odorant recognition principles.Sci Rep. 2015 Oct 9;5:14948. doi: 10.1038/srep14948. Sci Rep. 2015. PMID: 26449412 Free PMC article.
-
Modeling the Orthosteric Binding Site of the G Protein-Coupled Odorant Receptor OR5K1.J Chem Inf Model. 2023 Apr 10;63(7):2014-2029. doi: 10.1021/acs.jcim.2c00752. Epub 2023 Jan 25. J Chem Inf Model. 2023. PMID: 36696962 Free PMC article.
-
Transmembrane segment 3 of Drosophila melanogaster odorant receptor subunit 85b contributes to ligand-receptor interactions.J Biol Chem. 2010 Apr 16;285(16):11854-62. doi: 10.1074/jbc.M109.058321. Epub 2010 Feb 10. J Biol Chem. 2010. PMID: 20147286 Free PMC article.
-
Molecular Basis of Mammalian Odor Discrimination: A Status Report.J Agric Food Chem. 2018 Dec 26;66(51):13346-13366. doi: 10.1021/acs.jafc.8b04471. Epub 2018 Dec 7. J Agric Food Chem. 2018. PMID: 30453735 Review.
-
Deorphanizing vertebrate olfactory receptors: recent advances in odorant-response assays.Neurochem Int. 2007 Jul-Sep;51(2-4):132-9. doi: 10.1016/j.neuint.2007.05.020. Epub 2007 Jun 13. Neurochem Int. 2007. PMID: 17640771 Review.
Cited by
-
Lineage-Specific Class-A GPCR Dynamics Reflect Diverse Chemosensory Adaptations in Lophotrochozoa.Mol Biol Evol. 2025 Mar 5;42(3):msaf042. doi: 10.1093/molbev/msaf042. Mol Biol Evol. 2025. PMID: 39943858 Free PMC article.
-
Structural basis of odorant recognition by a human odorant receptor.Nature. 2023 Mar;615(7953):742-749. doi: 10.1038/s41586-023-05798-y. Epub 2023 Mar 15. Nature. 2023. PMID: 36922591 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

