Metabolomics Profiling of Nephrotic Syndrome towards Biomarker Discovery
- PMID: 36293474
- PMCID: PMC9603939
- DOI: 10.3390/ijms232012614
Metabolomics Profiling of Nephrotic Syndrome towards Biomarker Discovery
Abstract
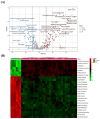
Nephrotic syndrome (NS) is a kidney illness characterized by excessive proteinuria, hypoalbuminemia, edema, and hyperlipidemia, which may lead to kidney failure and necessitate renal transplantation. End-stage renal disease, cardiovascular issues, and mortality are much more common in those with NS. Therefore, the present study aimed to identify potential new biomarkers associated with the pathogenesis and diagnosis of NS. The liquid chromatography-mass spectrometry (LC-MS) metabolomics approach was applied to profile the metabolome of human serum of patients with NS. A total of 176 metabolites were significantly altered in NS compared to the control. Arginine, proline, and tryptophan metabolism; arginine, phenylalanine, tyrosine, and tryptophan biosynthesis were the most common metabolic pathways dysregulated in NS. Furthermore, alanyl-lysine and isoleucyl-threonine had the highest discrimination between NS and healthy groups. The candidate biomarkers may lead to understanding the possible metabolic alterations associated with NS and serve as potential diagnostic biomarkers.
Keywords: biomarker; liquid chromatography–mass spectrometry; metabolomics; nephrotic syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gooding J.R., Agrawal S., McRitchie S., Acuff Z., Merchant M.L., Klein J.B., Smoyer W.E., Sumner S.J., Mahan J., Patel H. Predicting and defining steroid resistance in pediatric nephrotic syndrome using plasma metabolomics. Kidney Int. Rep. 2020;5:81–93. doi: 10.1016/j.ekir.2019.09.010. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

