Role of Glycolysis and Fatty Acid Synthesis in the Activation and T Cell-Modulating Potential of Dendritic Cells Stimulated with a TLR5-Ligand Allergen Fusion Protein
- PMID: 36293550
- PMCID: PMC9604253
- DOI: 10.3390/ijms232012695
Role of Glycolysis and Fatty Acid Synthesis in the Activation and T Cell-Modulating Potential of Dendritic Cells Stimulated with a TLR5-Ligand Allergen Fusion Protein
Abstract
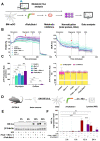
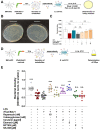
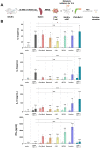
Trained immune responses, based on metabolic and epigenetic changes in innate immune cells, are de facto innate immune memory and, therefore, are of great interest in vaccine development. In previous studies, the recombinant fusion protein rFlaA:Betv1, combining the adjuvant and toll-like receptor (TLR)5-ligand flagellin (FlaA) and the major birch pollen allergen Bet v 1 into a single molecule, significantly suppressed allergic sensitization in vivo while also changing the metabolism of myeloid dendritic cells (mDCs). Within this study, the immune-metabolic effects of rFlaA:Betv1 during mDC activation were elucidated. In line with results for other well-characterized TLR-ligands, rFlaA:Betv1 increased glycolysis while suppressing oxidative phosphorylation to different extents, making rFlaA:Betv1 a suitable model to study the immune-metabolic effects of TLR-adjuvanted vaccines. In vitro pretreatment of mDCs with cerulenin (inhibitor of fatty acid biosynthesis) led to a decrease in both rFlaA:Betv1-induced anti-inflammatory cytokine Interleukin (IL) 10 and T helper cell type (TH) 1-related cytokine IL-12p70, while the pro-inflammatory cytokine IL 1β was unaffected. Interestingly, pretreatment with the glutaminase inhibitor BPTES resulted in an increase in IL-1β, but decreased IL-12p70 secretion while leaving IL-10 unchanged. Inhibition of the glycolytic enzyme hexokinase-2 by 2-deoxyglucose led to a decrease in all investigated cytokines (IL-10, IL-12p70, and IL-1β). Inhibitors of mitochondrial respiration had no effect on rFlaA:Betv1-induced IL-10 level, but either enhanced the secretion of IL-1β (oligomycin) or decreased IL-12p70 (antimycin A). In extracellular flux measurements, mDCs showed a strongly enhanced glycolysis after rFlaA:Betv1 stimulation, which was slightly increased after respiratory shutdown using antimycin A. rFlaA:Betv1-stimulated mDCs secreted directly antimicrobial substances in a mTOR- and fatty acid metabolism-dependent manner. In co-cultures of rFlaA:Betv1-stimulated mDCs with CD4+ T cells, the suppression of Bet v 1-specific TH2 responses was shown to depend on fatty acid synthesis. The effector function of rFlaA:Betv1-activated mDCs mainly relies on glycolysis, with fatty acid synthesis also significantly contributing to rFlaA:Betv1-mediated cytokine secretion, the production of antimicrobial molecules, and the modulation of T cell responses.
Keywords: Bet v 1; Warburg; allergy; flagellin; fusion protein; immune metabolism.
Conflict of interest statement
AG reports price money received from the German Society of Allergy and Clinical Immunology (DGAKI) and the Organizing Committee of the World Immune Regulation Meeting (WIRM) for abstracts and presentations relating to this publication. All other authors have no conflict of interest to declare.
Figures



References
-
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. - DOI - PMC - PubMed
-
- Schülke S., Fiedler A.-H., Junker A.-C., Flaczyk A., Wolfheimer S., Wangorsch A., Heinz A., Beckert H., Nagl B., Bohle B., et al. Critical Role of Mammalian Target of Rapamycin for IL-10 Dendritic Cell Induction by a Flagellin A Conjugate in Preventing Allergic Sensitization. J. Allergy Clin. Immunol. 2018;141:1786–1798.e11. doi: 10.1016/j.jaci.2017.07.002. - DOI - PubMed
-
- Lin Y.-J., Papp G., Miskey C., Fiedler A., Goretzki A., Wolfheimer S., Zimmermann J., Crauwels P., Ivics Z., van Zandbergen G., et al. The Flagellin:Allergen Fusion Protein RFlaA:Betv1 Induces a MyD88- and MAPK-Dependent Activation of Glucose Metabolism in Macrophages. Cells. 2021;10:2614. doi: 10.3390/cells10102614. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

