A Xanthohumol-Rich Hop Extract Diminishes Endotoxin-Induced Activation of TLR4 Signaling in Human Peripheral Blood Mononuclear Cells: A Study in Healthy Women
- PMID: 36293555
- PMCID: PMC9603845
- DOI: 10.3390/ijms232012702
A Xanthohumol-Rich Hop Extract Diminishes Endotoxin-Induced Activation of TLR4 Signaling in Human Peripheral Blood Mononuclear Cells: A Study in Healthy Women
Abstract
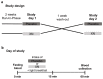
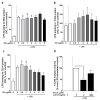
Infections with Gram-negative bacteria are still among the leading causes of infection-related deaths. Several studies suggest that the chalcone xanthohumol (XN) found in hop (Humulus lupulus) possesses anti-inflammatory effects. In a single-blinded, placebo controlled randomized cross-over design study we assessed if the oral intake of a single low dose of 0.125 mg of a XN derived through a XN-rich hop extract (75% XN) affects lipopolysaccharide (LPS)-induced immune responses in peripheral blood mononuclear cells (PBMCs) ex vivo in normal weight healthy women (n = 9) (clinicaltrials.gov: NCT04847193) and determined associated molecular mechanisms. LPS-stimulation of PBMCs isolated from participants 1 h after the intake of the placebo for 2 h resulted in a significant induction of pro-inflammatory cytokine release which was significantly attenuated when participants had consumed XN. The XN-dependent attenuation of proinflammatory cytokine release was less pronounced 6 h after the LPS stimulation while the release of sCD14 was significantly reduced at this timepoint. The LPS-dependent activation of hTLR4 transfected HEK293 cells was significantly and dose-dependently suppressed by the XN-rich hop extract which was attenuated when cells were co-challenged with sCD14. Taken together, our results suggest even a one-time intake of low doses of XN consumed in a XN-rich hop extract can suppress LPS-dependent stimulation of PBMCs and that this is related to the interaction of the hop compound with the CD14/TLR4 signaling cascade.
Keywords: CD14; LPS; TLR4; hop; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

