Effects of Sub-Minimum Inhibitory Concentrations of Imipenem and Colistin on Expression of Biofilm-Specific Antibiotic Resistance and Virulence Genes in Acinetobacter baumannii Sequence Type 1894
- PMID: 36293559
- PMCID: PMC9603859
- DOI: 10.3390/ijms232012705
Effects of Sub-Minimum Inhibitory Concentrations of Imipenem and Colistin on Expression of Biofilm-Specific Antibiotic Resistance and Virulence Genes in Acinetobacter baumannii Sequence Type 1894
Abstract
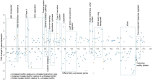
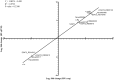
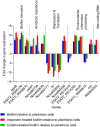
Antibiotics at suboptimal doses promote biofilm formation and the development of antibiotic resistance. The underlying molecular mechanisms, however, were not investigated. Here, we report the effects of sub-minimum inhibitory concentrations (sub-MICs) of imipenem and colistin on genes associated with biofilm formation and biofilm-specific antibiotic resistance in a multidrug-tolerant clinical strain of Acinetobacter baumannii Sequence Type (ST) 1894. Comparative transcriptome analysis was performed in untreated biofilm and biofilm treated with sub-MIC doses of imipenem and colistin. RNA sequencing data showed that 78 and 285 genes were differentially expressed in imipenem and colistin-treated biofilm cells, respectively. Among the differentially expressed genes (DEGs), 48 and 197 genes were upregulated exclusively in imipenem and colistin-treated biofilm cells, respectively. The upregulated genes included those encoding matrix synthesis (pgaB), multidrug efflux pump (novel00738), fimbrial proteins, and homoserine lactone synthase (AbaI). Upregulation of biofilm-associated genes might enhance biofilm formation when treated with sub-MICs of antibiotics. The downregulated genes include those encoding DNA gyrase (novel00171), 30S ribosomal protein S20 (novel00584), and ribosome releasing factor (RRF) were downregulated when the biofilm cells were treated with imipenem and colistin. Downregulation of these genes affects protein synthesis, which in turn slows down cell metabolism and makes biofilm cells more tolerant to antibiotics. In this investigation, we also found that 5 of 138 small RNAs (sRNAs) were differentially expressed in biofilm regardless of antibiotic treatment or not. Of these, sRNA00203 showed the highest expression levels in biofilm. sRNAs regulate gene expression and are associated with biofilm formation, which may in turn affect the expression of biofilm-specific antibiotic resistance. In summary, when biofilm cells were exposed to sub-MIC doses of colistin and imipenem, coordinated gene responses result in increased biofilm production, multidrug efflux pump expression, and the slowdown of metabolism, which leads to drug tolerance in biofilm. Targeting antibiotic-induced or repressed biofilm-specific genes represents a new strategy for the development of innovative and effective treatments for biofilm-associated infections caused by A. baumannii.
Keywords: Acinetobacter baumannii; RNA sequencing; antibiotic resistance; biofilm; colistin; imipenem; small RNA; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Harding C.M., Tracy E.N., Carruthers M.D., Rather P.N., Actis L.A., Munson R.S. Acinetobacter Baumannii Strain M2 Produces Type IV Pili Which Play a Role in Natural Transformation and Twitching Motility but Not Surface-Associated Motility. MBio. 2013;4:e00360-13. doi: 10.1128/mBio.00360-13. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

