DEPs Induce Local Ige Class Switching Independent of Their Ability to Stimulate iBALT de Novo Formation
- PMID: 36293642
- PMCID: PMC9603618
- DOI: 10.3390/ijerph192013063
DEPs Induce Local Ige Class Switching Independent of Their Ability to Stimulate iBALT de Novo Formation
Abstract
Background: Diesel exhaust particles (DEPs) are leading to a general increase in atopic diseases worldwide. However, it is still unknown whether DEPs induce systemic B-cell IgE class switching in secondary lymphoid organs or locally in the lungs in inducible bronchus-associated lymphoid tissue (iBALT). The aim of this work was to identify the exact site of DEP-mediated B-cell IgE class switching and pro-allergic antibody production.
Methods: We immunized BALB/c mice with different OVA doses (0.3 and 30 µg) intranasally in the presence and absence of two types of DEPs, SRM1650B and SRM2786. We used low (30 µg) and high (150 µg) DEP doses.
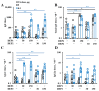
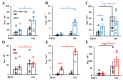
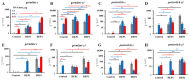
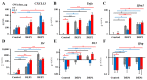
Results: Only a high DEP dose induced IgE production, regardless of the particle type. Local IgE class switching was stimulated upon treatment with both types of particles with both low and high OVA doses. Despite the similar ability of the two standard DEPs to stimulate IgE production, their ability to induce iBALT formation and growth was markedly different upon co-administration with low OVA doses.
Conclusions: DEP-induced local IgE class switching takes place in preexisting iBALTs independent of de novo iBALT formation, at least in the case of SRM1650B co-administered with low OVA doses.
Keywords: antibody production; antigen doses; diesel particulate matter; local Ig class switch; lungs; tertiary lymphoid structures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Tertiary lymphoid structure related B-cell IgE isotype switching and secondary lymphoid organ linked IgE production in mouse allergy model.BMC Immunol. 2020 Aug 7;21(1):45. doi: 10.1186/s12865-020-00376-7. BMC Immunol. 2020. PMID: 32767965 Free PMC article.
-
Roles of CD4+ and CD8+ T cells in adjuvant activity of diesel exhaust particles in mice.Int Arch Allergy Immunol. 2001 Apr;124(4):485-96. doi: 10.1159/000053784. Int Arch Allergy Immunol. 2001. PMID: 11340332
-
Combined nasal challenge with diesel exhaust particles and allergen induces In vivo IgE isotype switching.Am J Respir Cell Mol Biol. 1998 Sep;19(3):507-12. doi: 10.1165/ajrcmb.19.3.3143. Am J Respir Cell Mol Biol. 1998. PMID: 9730879
-
The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease.Allergy. 1997;52(38 Suppl):52-6; discussion 57-8. doi: 10.1111/j.1398-9995.1997.tb04871.x. Allergy. 1997. PMID: 9208060 Review.
-
Air pollution and allergy.J Investig Allergol Clin Immunol. 2000 Jan-Feb;10(1):5-10. J Investig Allergol Clin Immunol. 2000. PMID: 10780792 Review.
References
-
- Wang X., Wang Y., Bai Y., Wang P., Zhao Y. An overview of physical and chemical features of diesel exhaust particles. J. Energy Institute. 2018;92:1864–1888. doi: 10.1016/j.joei.2018.11.006. - DOI
-
- Yanagisava R., Takano H., Inoue K.-I., Sadakane K., Yoshino S., Yamaki K., Yoshikawa T., Hayakawa K. Components of diesel exhaust particles differentially affect Th1/Th2 response in a murine model of allergic airway inflammation. Clin. Exp. Allergy. 2006;36:386–395. doi: 10.1111/j.1365-2222.2006.02452.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

