SARS-CoV-2 Vaccines and Adverse Effects in Gynecology and Obstetrics: The First Italian Retrospective Study
- PMID: 36293746
- PMCID: PMC9603573
- DOI: 10.3390/ijerph192013167
SARS-CoV-2 Vaccines and Adverse Effects in Gynecology and Obstetrics: The First Italian Retrospective Study
Abstract
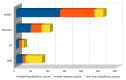
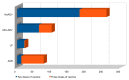
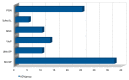
The most common effects reported by the Italian Medicine Agency following administration of SARS-CoV-2 vaccine are myalgia, soreness to the arm of inoculation, fever, and asthenia. To date, there are no specific and official reports registered by the Italian Medicine Agency on possible alterations of the menstrual cycle, or of the female reproductive system, following the vaccine. Actually, clinical experience showed a spread of transient adverse drug reactions of the menstrual cycle, following the administration of all COVID-19 vaccine types, both mRNA and Adenovirus vectored ones. In this work, we conducted the first retrospective study on Italian patients vaccinated for SARS-CoV-2 in the period between April 2021 and April 2022, to report the onset of menstrual changes after the vaccine in order to understand: etiology, duration of possible adverse effects, and the extent of the phenomenon. We recruited 100 women aged 18-45, vaccinated for SARS-CoV-2, who were asked to complete a questionnaire consisting of 12 multiple choice questions about the effects of the vaccine on the reproductive system. Thirty-seven of them received three doses of the vaccine, while the remaining 63 received two doses. Symptoms such as delayed menstruation and abnormal uterine bleeding (metrorrhagia, menometrorrhagia, and menorrhagia) were generally reported within the first three weeks of vaccination, especially after the second dose, with a percentage of 23% and 77%, respectively. These preliminary data suggest that this problem may be broader and deserving of further investigation in the future.
Keywords: SARS-CoV-2; anomalous uterine bleeding; gynecological and obstetric effects; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Evaluation of immunogenicity and reactogenicity of COVID-19 vaccines in pregnant women.Ultrasound Obstet Gynecol. 2022 Nov;60(5):673-680. doi: 10.1002/uog.26050. Ultrasound Obstet Gynecol. 2022. PMID: 36318630 Free PMC article.
-
Abnormal uterine bleeding diagnoses and care following COVID-19 vaccination.Am J Obstet Gynecol. 2024 May;230(5):540.e1-540.e13. doi: 10.1016/j.ajog.2024.01.006. Epub 2024 Jan 12. Am J Obstet Gynecol. 2024. PMID: 38219855
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Menstrual disturbances following COVID-19 vaccination: A probable puzzle about the role of endocrine and immune pathways.J Reprod Immunol. 2023 Aug;158:103952. doi: 10.1016/j.jri.2023.103952. Epub 2023 May 12. J Reprod Immunol. 2023. PMID: 37201456 Free PMC article. Review.
Cited by
-
COVID-19 Vaccine Knowledge, Attitude, Acceptance and Hesitancy among Pregnancy and Breastfeeding: Systematic Review of Hospital-Based Studies.Vaccines (Basel). 2023 Nov 7;11(11):1697. doi: 10.3390/vaccines11111697. Vaccines (Basel). 2023. PMID: 38006029 Free PMC article. Review.
-
Histopathological Patterns of Cutaneous Adverse Reaction to Anti-SARS-CoV-2 Vaccines: The Integrative Role of Skin Biopsy.Vaccines (Basel). 2023 Feb 9;11(2):397. doi: 10.3390/vaccines11020397. Vaccines (Basel). 2023. PMID: 36851273 Free PMC article. Review.
-
Impact of COVID-19 Vaccination on Menstrual Irregularities, Bleeding Patterns, and Cycle Duration: A Systematic Review and Meta-Analysis.Health Sci Rep. 2025 Jun 16;8(6):e70882. doi: 10.1002/hsr2.70882. eCollection 2025 Jun. Health Sci Rep. 2025. PMID: 40524715 Free PMC article. Review.
-
Safety evaluation of COVID-19 vaccination during early pregnancy: A single-center prospective cohort study of Chinese pregnant women.Hum Vaccin Immunother. 2023 Aug 1;19(2):2226995. doi: 10.1080/21645515.2023.2226995. Hum Vaccin Immunother. 2023. PMID: 37462023 Free PMC article.
-
Effects of Vitamin D on the Renin-Angiotensin System and Acute Childhood Pneumonia.Antibiotics (Basel). 2022 Nov 3;11(11):1545. doi: 10.3390/antibiotics11111545. Antibiotics (Basel). 2022. PMID: 36358201 Free PMC article.
References
-
- Actualité Point de Situation sur la Surveillance des Vaccins Contre la COVID-19—Période du 16/04/2021 au 22/04/2021. ANSM. 2021. [(accessed on 2 June 2021)]. Available online: https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-....
-
- Evans M.B., Alexander C., Barnard E., Ezzati M.M., Hill M.J., Hoyos L.R., Hariton E., Mikhael S., Penzias A., Center S.G.F. COVID-19 vaccine and infertility: Baseless claims and unfounded social media panic. [(accessed on 19 January 2021)];Fertil. Steril. 2021 23 Available online: https://www.fertstertdialog.com/posts/covid-19-vaccine-and-infertility-b....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

