A Participatory Framework for Plain Language Clinical Management Guideline Development
- PMID: 36294089
- PMCID: PMC9603256
- DOI: 10.3390/ijerph192013506
A Participatory Framework for Plain Language Clinical Management Guideline Development
Abstract
Background: Clinical management guidelines (CMGs) are decision support tools for patient care used by professionals, patients, and family caregivers. Since clinical experts develop numerous CMGs, their technical language hinders comprehension and access by nonmedical stakeholders. Additionally, the views of affected individuals and their families are often not incorporated into treatment guidelines. We developed an adequate methodology for addressing the needs and preferences of family and professional stakeholders regarding CMGs, a recently developed protocol for managing congenital disorders of glycosylation (CDG), a family of rare metabolic diseases. We used the CDG community and phosphomannomutase 2 (PMM2)-CDG CMGs as a pilot to test and implement our methodology.
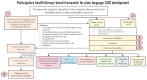
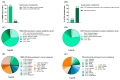
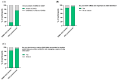
Results: We listened to 89 PMM2-CDG families and 35 professional stakeholders and quantified their CMG-related needs and preferences through an electronic questionnaire. Most families and professionals rated CMGs as relevant (86.5% and 94.3%, respectively), and valuable (84.3% and 94.3%, respectively) in CDG management. The most identified challenges were the lack of CMG awareness (50.6% of families) and the lack of plain language CMG (39.3% of professionals). Concordantly, among families, the most suggested solution was involving them in CMG development (55.1%), while professionals proposed adapting CMGs to include plain language (71.4%). Based on these results, a participatory framework built upon health literacy principles was created to improve CMG comprehension and accessibility. The outputs are six complementary CMG-related resources differentially adapted to the CDG community's needs and preferences, with a plain language PMM2-CDG CMG as the primary outcome. Additionally, the participants established a distribution plan to ensure wider access to all resources.
Conclusions: This empowering, people-centric methodology accelerates CMG development and accessibility to all stakeholders, ultimately improving the quality of life of individuals living with a specific condition and raising the possibility of application to other clinical guidelines.
Keywords: PMM2-CDG; clinical management guidelines; congenital disorders of glycosylation (CDG); health literacy; participatory medicine; people-centric; plain language; rare diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A Community-Based Participatory Framework to Co-Develop Patient Education Materials (PEMs) for Rare Diseases: A Model Transferable across Diseases.Int J Environ Res Public Health. 2023 Jan 5;20(2):968. doi: 10.3390/ijerph20020968. Int J Environ Res Public Health. 2023. PMID: 36673723 Free PMC article. Review.
-
Patient reported outcomes for phosphomannomutase 2 congenital disorder of glycosylation (PMM2-CDG): listening to what matters for the patients and health professionals.Orphanet J Rare Dis. 2022 Oct 29;17(1):398. doi: 10.1186/s13023-022-02551-y. Orphanet J Rare Dis. 2022. PMID: 36309700 Free PMC article.
-
Stakeholders' views on drug development: the congenital disorders of glycosylation community perspective.Orphanet J Rare Dis. 2022 Jul 30;17(1):303. doi: 10.1186/s13023-022-02460-0. Orphanet J Rare Dis. 2022. PMID: 35907899 Free PMC article.
-
International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: Diagnosis, treatment and follow up.J Inherit Metab Dis. 2019 Jan;42(1):5-28. doi: 10.1002/jimd.12024. J Inherit Metab Dis. 2019. PMID: 30740725
-
The road to successful people-centric research in rare diseases: the web-based case study of the Immunology and Congenital Disorders of Glycosylation questionnaire (ImmunoCDGQ).Orphanet J Rare Dis. 2022 Mar 24;17(1):134. doi: 10.1186/s13023-022-02286-w. Orphanet J Rare Dis. 2022. PMID: 35331276 Free PMC article.
Cited by
-
A Community-Based Participatory Framework to Co-Develop Patient Education Materials (PEMs) for Rare Diseases: A Model Transferable across Diseases.Int J Environ Res Public Health. 2023 Jan 5;20(2):968. doi: 10.3390/ijerph20020968. Int J Environ Res Public Health. 2023. PMID: 36673723 Free PMC article. Review.
-
A community-centric model for conference co-creation: the world conference on CDG for patients, families and professionals.Res Involv Engagem. 2024 Oct 23;10(1):107. doi: 10.1186/s40900-024-00641-8. Res Involv Engagem. 2024. PMID: 39443988 Free PMC article.
-
Mapping the diagnostic odyssey of congenital disorders of glycosylation (CDG): insights from the community.Orphanet J Rare Dis. 2024 Nov 1;19(1):407. doi: 10.1186/s13023-024-03389-2. Orphanet J Rare Dis. 2024. PMID: 39482754 Free PMC article.
References
-
- Field M.J., Lohr K.N., editors. Clinical Practice Guidelines: Directions for a New Program. National Academies Press; Washington, DC, USA: 1990. - PubMed
-
- Alonso-Coello P., Irfan A., Solà I., Gich I., Delgado-Noguera M.F., Rigau D., Tort S., Bonfill X., Burgers J., Schunemann H. The quality of clinical practice guidelines over the last two decades: A systematic review of guideline appraisal studies. BMJ Qual. Saf. Health Care. 2010;19:e58. doi: 10.1136/qshc.2010.042077. - DOI - PubMed
-
- Vega M.B., Benavent P.G., de Val M.S.I., Fernández C.M., Remón L.P., Sanchéz J.I.M., Diez M.P.B., Mérez M.P.C., Armesto S.G., del Pozo S.V.F., et al. Methodological Handbooks & Toolkit for Clinical Practice Guidelines and Clinical Decision Support Tools for Rare or Low Prevalence and Complex Diseases. European Reference Networks. 2020. [(accessed on 15 September 2022)]. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rj....
-
- Steeb T., Hayani K.M., Förster P., Liegl R., Toussaint F., Schlaak M., Berking C., Heppt M.V. Guidelines for uveal melanoma: A critical appraisal of systematically identified guidelines using the AGREE II and AGREE-REX instrument. J. Cancer Res. Clin. Oncol. 2020;146:1079–1088. doi: 10.1007/s00432-020-03141-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

