Evaluation of Fecal Calprotectin, Serum C-Reactive Protein, Erythrocyte Sedimentation Rate, Seromucoid and Procalcitonin in the Diagnostics and Monitoring of Crohn's Disease in Children
- PMID: 36294408
- PMCID: PMC9604851
- DOI: 10.3390/jcm11206086
Evaluation of Fecal Calprotectin, Serum C-Reactive Protein, Erythrocyte Sedimentation Rate, Seromucoid and Procalcitonin in the Diagnostics and Monitoring of Crohn's Disease in Children
Abstract
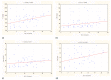
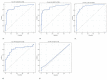
Background: The development of diagnostic and monitoring algorithms for Crohn’s disease based on non-invasive methods is of particular importance in children and is the subject of many studies. Objectives: Evaluate the usefulness of fecal calprotectin, serum C-reactive protein, erythrocyte sedimentation rate, seromucoid and procalcitonin in the differential diagnosis of non-inflammatory gastrointestinal tract diseases and Crohn’s disease in children and their usefulness in determining the phenotype of Crohn’s disease. Material and methods: Forty-seven children with non-inflammatory gastrointestinal tract diseases and fifty-four with Crohn’s disease were enrolled. Clinical and endoscopic activity was evaluated based on the Pediatric Crohn’s Disease Activity Index (PCDAI) and the Simple Endoscopic Score for Crohn’s Disease (SES-CD). Results: Fecal calprotectin, C-reactive protein, erythrocyte sedimentation rate and seromucoid were significantly higher in children with Crohn’s disease than in controls (p < 0.001). Fecal calprotectin correlated with clinical and endoscopic activity according to the Pediatric Crohn’s Disease Activity Index (r = 0.338; p = 0.012) and the Simple Endoscopic Score for Crohn’s Disease (r = 0.428; p = 0.001). Non-invasive biomarkers did not correlate with the location and clinical manifestation of Crohn’s disease. Conclusions: Fecal calprotectin, C-reactive protein, erythrocyte sedimentation rate and seromucoid are useful in the differentiation of Crohn’s disease from non-inflammatory gastrointestinal tract diseases in children and in monitoring the clinical course of Crohn’s disease, but not in evaluating activity and phenotype of the disease.
Keywords: activity; biomarkers; diagnosis; monitoring; non-invasive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI.Am J Gastroenterol. 2010 Jan;105(1):162-9. doi: 10.1038/ajg.2009.545. Epub 2009 Sep 15. Am J Gastroenterol. 2010. PMID: 19755969
-
Serum calprotectin as a biomarker for Crohn's disease.J Crohns Colitis. 2013 Dec;7(12):e678-83. doi: 10.1016/j.crohns.2013.06.008. Epub 2013 Jul 9. J Crohns Colitis. 2013. PMID: 23845231 Clinical Trial.
-
The combination of fecal calprotectin with ESR, CRP and albumin discriminates more accurately children with Crohn's disease.Adv Med Sci. 2019 Mar;64(1):9-14. doi: 10.1016/j.advms.2018.08.001. Epub 2018 Sep 18. Adv Med Sci. 2019. PMID: 30237086
-
What is the role of C-reactive protein and fecal calprotectin in evaluating Crohn's disease activity?Best Pract Res Clin Gastroenterol. 2019 Feb-Apr;38-39:101602. doi: 10.1016/j.bpg.2019.02.004. Epub 2019 Feb 22. Best Pract Res Clin Gastroenterol. 2019. PMID: 31327404 Review.
-
Cannabis for the treatment of Crohn's disease.Cochrane Database Syst Rev. 2018 Nov 8;11(11):CD012853. doi: 10.1002/14651858.CD012853.pub2. Cochrane Database Syst Rev. 2018. PMID: 30407616 Free PMC article.
Cited by
-
Combining site-specific gut microbiome and mycobiome profiling with clinical indicators for effective management of pediatric Crohn's disease.iScience. 2025 Jul 18;28(8):113160. doi: 10.1016/j.isci.2025.113160. eCollection 2025 Aug 15. iScience. 2025. PMID: 40822348 Free PMC article.
References
-
- Setty M., Russell G.H., Bousvaros A. Clinical manifestations of Crohn disease in children and adolescents. In: Hoppin A.G., Post T.W., editors. UpToDate: Industry-Leading Clinical Decision Support. UpToDate; Waltham, MA, USA: 2016. [(accessed on 1 December 2016)]. Available online: http://www.uptodate.com/home.
-
- Gomollón F., Dignass A., Annese V., Tilg H., Van Assche G., Lindsay J.O., Peyrin-Biroulet L., Cullen G.J., Daperno M., Kucharzik T., et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. - DOI - PubMed
-
- Schoepfer A., Dehlavi M., Fournier N., Safroneeva E., Straumann A., Pittet V., Peyrin-Biroulet L., Michetti P., Rogler G., Vavricka S., et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am. J. Gastroenterol. 2013;108:1744–1753. doi: 10.1038/ajg.2013.248. - DOI - PubMed
-
- Ruemmele F., Veres G., Kolho K., Griffiths A., Levine A., Escher J., Amil Dias J., Barabino A., Braegger C.P., Bronskyet J., et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis. 2014;8:1179–1207. doi: 10.1016/j.crohns.2014.04.005. - DOI - PubMed
-
- Levine A., Griffiths A., Markowitz J., Wilson D., Turner D., Russell R., Fell J., Ruemmele F.M., Walters T., Sherlock M., et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel. Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

