Association between Germline Single-Nucleotide Variants in ADME Genes and Major Molecular Response to Imatinib in Chronic Myeloid Leukemia Patients
- PMID: 36294538
- PMCID: PMC9604607
- DOI: 10.3390/jcm11206217
Association between Germline Single-Nucleotide Variants in ADME Genes and Major Molecular Response to Imatinib in Chronic Myeloid Leukemia Patients
Abstract
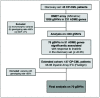
Imatinib is the most common first-line tyrosine kinase inhibitor (TKI) used to treat chronic-phase chronic myeloid leukemia (CP-CML). However, only a proportion of patients achieve major molecular response (MMR), so there is a need to find biological factors that aid the selection of the optimal therapeutic strategy (imatinib vs. more potent second-generation TKIs). The aim of this retrospective study was to understand the contribution of germline single-nucleotide variants (gSNVs) in the achievement of MMR with imatinib. In particular, a discovery cohort including 45 CP-CML patients was analyzed through the DMET array, which interrogates 1936 variants in 231 genes related to the absorption, distribution, metabolism and excretion (ADME) process. Variants statistically significant in the discovery cohort were then tested in an extended and independent cohort of 137 CP-CML patients. Finally, a total of 7 gSNVs (ABCG1-rs492338, ABCB11-rs496550, ABCB11-rs497692, CYP2D6-rs1135840, CYP11B1-rs7003319, MAT1A-rs4934027 and SLC22A1-rs628031) and one haplotype in the ABCB11 gene were significantly associated with the achievement of MMR with first-line imatinibtreatment. In conclusion, we identified a genetic signature of response to imatinib in CP-CML patients that could be useful in selecting those patients that may benefit from starting imatinib as first-line therapy, therefore avoiding the toxicity related to second-generation TKIs.
Keywords: chronic myeloid leukemia; imatinib; major molecular response; single-nucleotide polymorphisms.
Conflict of interest statement
B.X. and L.Z. have received a grant from Novartis. F.F.M. has received a grant from Incyte. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results. The rest of the authors declare no conflict of interest.
Figures

Similar articles
-
Dasatinib rapidly induces deep molecular response in chronic-phase chronic myeloid leukemia patients who achieved major molecular response with detectable levels of BCR-ABL1 transcripts by imatinib therapy.Int J Clin Oncol. 2017 Oct;22(5):972-979. doi: 10.1007/s10147-017-1141-y. Epub 2017 May 26. Int J Clin Oncol. 2017. PMID: 28550414 Free PMC article. Clinical Trial.
-
An exploratory study by DMET array identifies a germline signature associated with imatinib response in gastrointestinal stromal tumor.Pharmacogenomics J. 2019 Aug;19(4):390-400. doi: 10.1038/s41397-018-0050-4. Epub 2018 Sep 20. Pharmacogenomics J. 2019. PMID: 30237583
-
OCT1 genetic variants are associated with long term outcomes in imatinib treated chronic myeloid leukemia patients.Eur J Haematol. 2014 Apr;92(4):283-8. doi: 10.1111/ejh.12235. Epub 2013 Dec 18. Eur J Haematol. 2014. PMID: 24215657
-
First-line imatinib vs second- and third-generation TKIs for chronic-phase CML: a systematic review and meta-analysis.Blood Adv. 2020 Jun 23;4(12):2723-2735. doi: 10.1182/bloodadvances.2019001329. Blood Adv. 2020. PMID: 32559295 Free PMC article.
-
A critical review of trials of first-line BCR-ABL inhibitor treatment in patients with newly diagnosed chronic myeloid leukemia in chronic phase.Clin Lymphoma Myeloma Leuk. 2013 Dec;13(6):646-56. doi: 10.1016/j.clml.2013.05.012. Epub 2013 Oct 1. Clin Lymphoma Myeloma Leuk. 2013. PMID: 24095296 Free PMC article. Review.
Cited by
-
A Comprehensive Metabolism-Related Gene Signature Predicts the Survival of Patients with Acute Myeloid Leukemia.Genes (Basel). 2023 Dec 31;15(1):63. doi: 10.3390/genes15010063. Genes (Basel). 2023. PMID: 38254953 Free PMC article.
References
-
- Hochhaus A., Baccarani M., Silver R.T., Schiffer C., Apperley J.F., Cervantes F., Clark R.E., Cortes J.E., Deininger M.W., Guilhot F., et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984. doi: 10.1038/s41375-020-0776-2. - DOI - PMC - PubMed
-
- Etienne G., Dulucq S., Huguet F., Schmitt A., Lascaux A., Hayette S., Fort M.P., Sujobert P., Bijou F., Morisset S., et al. Incidence and outcome of BCR-ABL mutated chronic myeloid leukemia patients who failed to tyrosine kinase inhibitors. Cancer Med. 2019;8:5173–5182. doi: 10.1002/cam4.2410. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

