Association between Germline Single-Nucleotide Variants in ADME Genes and Major Molecular Response to Imatinib in Chronic Myeloid Leukemia Patients
- PMID: 36294538
- PMCID: PMC9604607
- DOI: 10.3390/jcm11206217
Association between Germline Single-Nucleotide Variants in ADME Genes and Major Molecular Response to Imatinib in Chronic Myeloid Leukemia Patients
Abstract
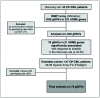
Imatinib is the most common first-line tyrosine kinase inhibitor (TKI) used to treat chronic-phase chronic myeloid leukemia (CP-CML). However, only a proportion of patients achieve major molecular response (MMR), so there is a need to find biological factors that aid the selection of the optimal therapeutic strategy (imatinib vs. more potent second-generation TKIs). The aim of this retrospective study was to understand the contribution of germline single-nucleotide variants (gSNVs) in the achievement of MMR with imatinib. In particular, a discovery cohort including 45 CP-CML patients was analyzed through the DMET array, which interrogates 1936 variants in 231 genes related to the absorption, distribution, metabolism and excretion (ADME) process. Variants statistically significant in the discovery cohort were then tested in an extended and independent cohort of 137 CP-CML patients. Finally, a total of 7 gSNVs (ABCG1-rs492338, ABCB11-rs496550, ABCB11-rs497692, CYP2D6-rs1135840, CYP11B1-rs7003319, MAT1A-rs4934027 and SLC22A1-rs628031) and one haplotype in the ABCB11 gene were significantly associated with the achievement of MMR with first-line imatinibtreatment. In conclusion, we identified a genetic signature of response to imatinib in CP-CML patients that could be useful in selecting those patients that may benefit from starting imatinib as first-line therapy, therefore avoiding the toxicity related to second-generation TKIs.
Keywords: chronic myeloid leukemia; imatinib; major molecular response; single-nucleotide polymorphisms.
Conflict of interest statement
B.X. and L.Z. have received a grant from Novartis. F.F.M. has received a grant from Incyte. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results. The rest of the authors declare no conflict of interest.
Figures

References
-
- Hochhaus A., Baccarani M., Silver R.T., Schiffer C., Apperley J.F., Cervantes F., Clark R.E., Cortes J.E., Deininger M.W., Guilhot F., et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–984. doi: 10.1038/s41375-020-0776-2. - DOI - PMC - PubMed
-
- Etienne G., Dulucq S., Huguet F., Schmitt A., Lascaux A., Hayette S., Fort M.P., Sujobert P., Bijou F., Morisset S., et al. Incidence and outcome of BCR-ABL mutated chronic myeloid leukemia patients who failed to tyrosine kinase inhibitors. Cancer Med. 2019;8:5173–5182. doi: 10.1002/cam4.2410. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

