Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants
- PMID: 36294540
- PMCID: PMC9605537
- DOI: 10.3390/jcm11206218
Chondral/Desmal Osteogenesis in 3D Spheroids Sensitized by Psychostimulants
Abstract
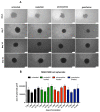
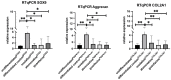
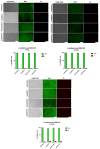
Attention deficit hyperactivity disorder (ADHD) affects 6.4 million children in the United States of America. Children and adolescents, the main consumers of ADHD medication, are in the bone growth phase, which extends over a period of up to two decades. Thus, impaired proliferation and maturation of chondrocytes and osteoblasts can result in impaired bone formation. The aim of this study is to investigate, for the first time, the effects of the ADHD-medication modafinil, atomoxetine and guanfacine on bone growth and repair in vitro. Using two-dimensional and three-dimensional cell models, we investigated the chondrogenic/osteogenic differentiation, proliferation and viability of human mesenchymal progenitor cells. Real-time cell proliferation was measured by xCELLigence. Live/dead staining and size measurement of hMSC- and MG63 monolayer and spheroids were performed after administration of therapeutic plasma concentrations of modafinil, atomoxetine and guanfacine. Chondrogenic differentiation was quantified by RTqPCR. The chondrogenic and osteogenic differentiation was evaluated by histological cryo-sections. Modafinil, atomoxetine and guanfacine reduced chondrogenic and osteogenic differentiation terms of transcript expression and at the histological level. Cell viability of the MG63- and hMSC monolayer was not impeded by ADHD-medication. Our in vitro results indicate that modafinil, atomoxetine and guanfacine may impair chondrogenic and osteogenic differentiation in a 3D model reflecting the in vivo physiologic condition.
Keywords: bone defect; cell spheroids ADHD; cell viability; hMSCs; osteogenic/chondrogenic differentiation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
-
- Science Direct: Endochondral Ossification. [(accessed on 16 June 2022)]. Available online: https://www.sciencedirect.com/topics/veterinary-science-and-veterinary-m....
LinkOut - more resources
Full Text Sources

