Reactivation of Coccidioidomycosis in a Mouse Model of Asymptomatic Controlled Disease
- PMID: 36294555
- PMCID: PMC9605249
- DOI: 10.3390/jof8100991
Reactivation of Coccidioidomycosis in a Mouse Model of Asymptomatic Controlled Disease
Abstract
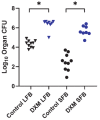
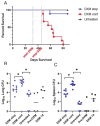
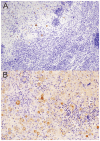
The majority of human coccidioidomycosis infections are asymptomatic or self-limited but may have sequestered spherules in highly structured granulomas. Under immunosuppression, reactivation of fungal growth can result in severe disease. B6D2F1 mice asymptomatically infected with C. posadasii strain 1038 were immunosuppressed with dexamethasone (DXM) in drinking water. Treated mice died 16−25 days later, while untreated mice survived (p < 0.001). Flow cytometry of lung granulomas on days 5, 10, 15, and 20 of DXM treatment showed immune cell populations decreased 0.5−1 log compared with untreated mice though neutrophils and CD19+IgD−IgM− cells rebounded by day 20. Histopathology demonstrated loss of granuloma structure by day 5 and increasing spherules through day 20. On day 20, T-cells were nearly absent and disorganized pyogranulomatous lesions included sheets of plasma cells and innumerable spherules. Mice given DXM for 14 days then stopped (DXM stop) survived 6 weeks (9/10). Lung fungal burdens were significantly lower (p = 0.0447) than mice that continued treatment (DXM cont) but higher than untreated mice. Histopathologically, DXM stop mice did not redevelop controlled granulomas by sacrifice, though T-cells were densely scattered throughout the lesions. This demonstrates a mouse model suitable for further study to understand the immunologic components responsible for maintenance control of coccidioidomycosis.
Keywords: Coccidioides; granuloma; immunosuppression; mice; reactivation.
Conflict of interest statement
None of the authors has a conflict of interest.
Figures


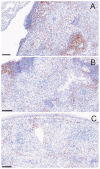
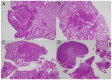

Similar articles
-
A Chronic Murine Disease Model of Coccidioidomycosis Using Coccidioides posadasii, Strain 1038.J Infect Dis. 2021 Jan 4;223(1):166-173. doi: 10.1093/infdis/jiaa419. J Infect Dis. 2021. PMID: 32658292 Free PMC article.
-
The Novel Fungal Cyp51 Inhibitor VT-1598 Is Efficacious in Experimental Models of Central Nervous System Coccidioidomycosis Caused by Coccidioides posadasii and Coccidioides immitis.Antimicrob Agents Chemother. 2018 Mar 27;62(4):e02258-17. doi: 10.1128/AAC.02258-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29437615 Free PMC article.
-
The Orotomide Olorofim Is Efficacious in an Experimental Model of Central Nervous System Coccidioidomycosis.Antimicrob Agents Chemother. 2018 Aug 27;62(9):e00999-18. doi: 10.1128/AAC.00999-18. Print 2018 Sep. Antimicrob Agents Chemother. 2018. PMID: 29941638 Free PMC article.
-
Immune Response to Coccidioidomycosis and the Development of a Vaccine.Microorganisms. 2017 Mar 16;5(1):13. doi: 10.3390/microorganisms5010013. Microorganisms. 2017. PMID: 28300772 Free PMC article. Review.
-
Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci. 2007 Sep;1111:301-14. doi: 10.1196/annals.1406.049. Epub 2007 Mar 15. Ann N Y Acad Sci. 2007. PMID: 17363434 Review.
Cited by
-
The Known and Unknown "Knowns" of Human Susceptibility to Coccidioidomycosis.J Fungi (Basel). 2024 Mar 28;10(4):256. doi: 10.3390/jof10040256. J Fungi (Basel). 2024. PMID: 38667927 Free PMC article. Review.
-
From soil to clinic: current advances in understanding Coccidioides and coccidioidomycosis.Microbiol Mol Biol Rev. 2024 Dec 18;88(4):e0016123. doi: 10.1128/mmbr.00161-23. Epub 2024 Oct 4. Microbiol Mol Biol Rev. 2024. PMID: 39365073 Review.
-
Coccidioidomycosis Granulomas Informed by Other Diseases: Advancements, Gaps, and Challenges.J Fungi (Basel). 2023 Jun 9;9(6):650. doi: 10.3390/jof9060650. J Fungi (Basel). 2023. PMID: 37367586 Free PMC article. Review.
-
Spatiotemporal analysis of lung immune dynamics in lethal Coccidioides posadasii infection.mBio. 2025 May 14;16(5):e0256224. doi: 10.1128/mbio.02562-24. Epub 2024 Nov 29. mBio. 2025. PMID: 39611685 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

