Online Biomass Monitoring Enables Characterization of the Growth Pattern of Aspergillus fumigatus in Liquid Shake Conditions
- PMID: 36294578
- PMCID: PMC9605507
- DOI: 10.3390/jof8101013
Online Biomass Monitoring Enables Characterization of the Growth Pattern of Aspergillus fumigatus in Liquid Shake Conditions
Abstract
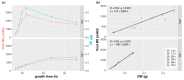
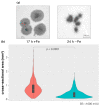
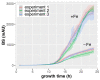
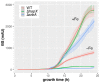
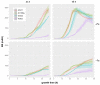
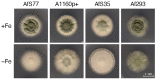
Numerous filamentous fungal species are extensively studied due to their role as model organisms, workhorses in biotechnology, or as pathogens for plants, animals, and humans. Growth studies are mainly carried out on solid media. However, studies concerning gene expression, biochemistry, or metabolism are carried out usually in liquid shake conditions, which do not correspond to the growth pattern on solid media. The reason for this practice is the problem of on-line growth monitoring of filamentous fungal species, which usually form pellets in liquid shake cultures. Here, we compared the time-consuming and tedious process of dry-weight determination of the mold Aspergillus fumigatus with online monitoring of biomass in liquid shake culture by the parallelizable CGQ ("cell growth quantifier"), which implements dynamic biomass determination by backscattered light measurement. The results revealed a strong correlation of CGQ-mediated growth monitoring and classical biomass measurement of A. fumigatus grown over a time course. Moreover, CGQ-mediated growth monitoring displayed the difference in growth of A. fumigatus in response to the limitation of iron or nitrogen as well as the growth defects of previously reported mutant strains (ΔhapX, ΔsrbA). Furthermore, the frequently used wild-type strain Af293 showed largely decreased and delayed growth in liquid shake cultures compared to other strains (AfS77, A1160p+, AfS35). Taken together, the CGQ allows for robust, automated biomass monitoring of A. fumigatus during liquid shake conditions, which largely facilitates the characterization of the growth pattern of filamentous fungal species.
Keywords: Aspergillus fumigatus; backscatter; biomass monitoring; bioprocess automation; flask culture; fungi; liquid shake culture; molds; online monitoring.
Conflict of interest statement
B.L. is an employee of Scientific Bioprocessing (SBI) developing the CGQ system. The other authors declare that they have no competing interest.
Figures





Similar articles
-
Parallelised online biomass monitoring in shake flasks enables efficient strain and carbon source dependent growth characterisation of Saccharomyces cerevisiae.Microb Cell Fact. 2016 Jul 25;15(1):127. doi: 10.1186/s12934-016-0526-3. Microb Cell Fact. 2016. PMID: 27455954 Free PMC article.
-
Quantitative Monitoring of Mycelial Growth of Aspergillus fumigatus in Liquid Culture by Optical Density.Microbiol Spectr. 2022 Feb 23;10(1):e0006321. doi: 10.1128/spectrum.00063-21. Epub 2022 Jan 5. Microbiol Spectr. 2022. PMID: 34985327 Free PMC article.
-
The Heterotrimeric Transcription Factor CCAAT-Binding Complex and Ca2+-CrzA Signaling Reversely Regulate the Transition between Fungal Hyphal Growth and Asexual Reproduction.mBio. 2021 Dec 21;12(6):e0300721. doi: 10.1128/mBio.03007-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781745 Free PMC article.
-
Practices of shake-flask culture and advances in monitoring CO2 and O2.Appl Microbiol Biotechnol. 2018 May;102(10):4279-4289. doi: 10.1007/s00253-018-8922-8. Epub 2018 Mar 26. Appl Microbiol Biotechnol. 2018. PMID: 29582104 Review.
-
Pitfalls in Early Bioprocess Development Using Shake Flask Cultivations.Eng Life Sci. 2025 Jan 28;25(1):e70001. doi: 10.1002/elsc.70001. eCollection 2025 Jan. Eng Life Sci. 2025. PMID: 39877379 Free PMC article. Review.
Cited by
-
Squalene production under oxygen limitation by Schizochytrium sp. S31 in different cultivation systems.Appl Microbiol Biotechnol. 2024 Feb 13;108(1):201. doi: 10.1007/s00253-024-13051-3. Appl Microbiol Biotechnol. 2024. PMID: 38349390 Free PMC article.
-
The Transcription Factors AcuK and AcuM Influence Siderophore Biosynthesis of Aspergillus fumigatus.J Fungi (Basel). 2024 Apr 30;10(5):327. doi: 10.3390/jof10050327. J Fungi (Basel). 2024. PMID: 38786682 Free PMC article.
References
-
- Meyer V., Andersen M.R., Brakhage A.A., Braus G.H., Caddick M.X., Cairns T.C., de Vries R.P., Haarmann T., Hansen K., Hertz-Fowler C., et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016;3:6. doi: 10.1186/s40694-016-0024-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

