Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans
- PMID: 36294593
- PMCID: PMC9604646
- DOI: 10.3390/jof8101028
Overexpression of the White Opaque Switching Master Regulator Wor1 Alters Lipid Metabolism and Mitochondrial Function in Candida albicans
Abstract
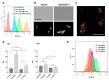
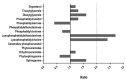
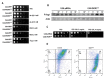
Candida albicans is a commensal yeast that inhabits the gastrointestinal tract of humans; increased colonization of this yeast in this niche has implicated the master regulator of the white-opaque transition, Wor1, by mechanisms not completely understood. We have addressed the role that this transcription factor has on commensalism by the characterization of strains overexpressing this gene. We show that WOR1 overexpression causes an alteration of the total lipid content of the fungal cell and significantly alters the composition of structural and reserve molecular species lipids as determined by lipidomic analysis. These cells are hypersensitive to membrane-disturbing agents such as SDS, have increased tolerance to azoles, an augmented number of peroxisomes, and increased phospholipase activity. WOR1 overexpression also decreases mitochondrial activity and results in altered susceptibility to certain oxidants. All together, these changes reflect drastic alterations in the cellular physiology that facilitate adaptation to the gastrointestinal tract environment.
Keywords: Candida albicans; Wor1; commensalism; gut; lipid; mitochondrial activity; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
The Glyoxylate Cycle Is Involved in White-Opaque Switching in Candida albicans.J Fungi (Basel). 2021 Jun 24;7(7):502. doi: 10.3390/jof7070502. J Fungi (Basel). 2021. PMID: 34202465 Free PMC article.
-
The defective gut colonization of Candida albicans hog1 MAPK mutants is restored by overexpressing the transcriptional regulator of the white opaque transition WOR1.Virulence. 2023 Dec;14(1):2174294. doi: 10.1080/21505594.2023.2174294. Virulence. 2023. PMID: 36760104 Free PMC article.
-
Wor1-regulated ferroxidases contribute to pigment formation in opaque cells of Candida albicans.FEBS Open Bio. 2021 Mar;11(3):598-621. doi: 10.1002/2211-5463.13070. Epub 2021 Feb 18. FEBS Open Bio. 2021. PMID: 33350590 Free PMC article.
-
Regulation of white-opaque switching in Candida albicans.Med Microbiol Immunol. 2010 Aug;199(3):165-72. doi: 10.1007/s00430-010-0147-0. Med Microbiol Immunol. 2010. PMID: 20390300 Review.
-
Adaptation of Candida albicans to commensalism in the gut.Future Microbiol. 2016;11(4):567-83. doi: 10.2217/fmb.16.1. Epub 2016 Apr 12. Future Microbiol. 2016. PMID: 27070839 Review.
References
-
- Youssef E.G., Zhang L., Alkhazraji S., Gebremariam T., Singh S., Yount N.Y., Yeaman M.R., Uppuluri P., Ibrahim A.S. Monoclonal IgM Antibodies Targeting Candida albicans Hyr1 Provide Cross-Kingdom Protection against Gram-Negative Bacteria. Front. Immunol. 2020;11:76. doi: 10.3389/fimmu.2020.00076. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

