Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen
- PMID: 36294634
- PMCID: PMC9605252
- DOI: 10.3390/jof8101069
Cryptococcus neoformans Infection in the Central Nervous System: The Battle between Host and Pathogen
Abstract
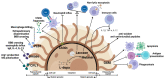
Cryptococcus neoformans (C. neoformans) is a pathogenic fungus with a global distribution. Humans become infected by inhaling the fungus from the environment, and the fungus initially colonizes the lungs. If the immune system fails to contain C. neoformans in the lungs, the fungus can disseminate to the blood and invade the central nervous system, resulting in fatal meningoencephalitis particularly in immunocompromised individuals including HIV/AIDS patients. Following brain invasion, C. neoformans will encounter host defenses involving resident as well as recruited immune cells in the brain. To overcome host defenses, C. neoformans possesses multiple virulence factors capable of modulating immune responses. The outcome of the interactions between the host and C. neoformans will determine the disease progression. In this review, we describe the current understanding of how C. neoformans migrates to the brain across the blood-brain barrier, and how the host immune system responds to the invading organism in the brain. We will also discuss the virulence factors that C. neoformans uses to modulate host immune responses.
Keywords: Cryptococcus neoformans; NK cells; T cells; Trojan horse; brain invasion; central nervous system; chitin; cryptococcosis; cytokines; fungal dissemination; fungal pathogenesis; fungus; laccase; macrophages; melanin; meningoencephalitis; microglia; monocytes; paracellular crossing; phospholipase B1; polysaccharide capsule; transcytosis; urease; virulence factors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Perfect J.R., Dismukes W.E., Dromer F., Goldman D.L., Graybill J.R., Hamill R.J., Harrison T.S., Larsen R.A., Lortholary O., Nguyen M.H., et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

