Guanidine-Containing Antifungal Agents against Human-Relevant Fungal Pathogens (2004-2022)-A Review
- PMID: 36294650
- PMCID: PMC9605545
- DOI: 10.3390/jof8101085
Guanidine-Containing Antifungal Agents against Human-Relevant Fungal Pathogens (2004-2022)-A Review
Abstract
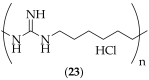
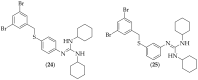
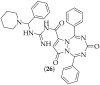
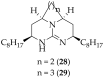
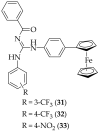
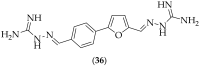
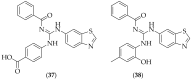
The guanidine moiety is typically a highly basic group, and can be found in a wide variety of drugs, such as zanamivir (Relenza) and metformin (Fortamet), as well as in biologically active compounds for numerous disease areas, including central nervous system (CNS) diseases and chemotherapeutics. This review will focus on antifungal agents which contain at least one guanidine group, for the treatment of human-related fungal pathogens, described in the literature between 2004 and 2022. These compounds include small molecules, steroids, polymers, metal complexes, sesquiterpenes, natural products, and polypeptides. It shall be made clear that a diverse range of guanidine-containing derivatives have been published in the literature and have antifungal activity, including efficacy in in vivo experiments.
Keywords: antifungal agent; guanidine; metal complex; natural product; polymer; polypeptide; sesquiterpene; small molecule; steroid.
Conflict of interest statement
The author declares no conflict of interest.
Figures







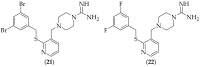





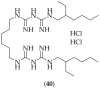


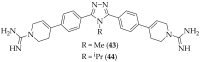












References
-
- Hussain Z., Khalaf M., Ibraheem H., Yousif E. Application of guanidine in pharmaceutical field. J. Chem. Pharm. 2016;8:127–129.
Publication types
LinkOut - more resources
Full Text Sources

