Alterations in Homologous Recombination-Related Genes and Distinct Platinum Response in Metastatic Triple-Negative Breast Cancers: A Subgroup Analysis of the ProfiLER-01 Trial
- PMID: 36294734
- PMCID: PMC9604780
- DOI: 10.3390/jpm12101595
Alterations in Homologous Recombination-Related Genes and Distinct Platinum Response in Metastatic Triple-Negative Breast Cancers: A Subgroup Analysis of the ProfiLER-01 Trial
Abstract
Background: a specific subset of metastatic triple-negative breast cancers (mTNBC) is characterized by homologous recombination deficiency (HRD), leading to enhanced sensitivity to platinum-based chemotherapy. Apart from mutations in BRCA1/2 genes, the evaluation of other HRD-related alterations has been limited to date. As such, we analyzed data from mTNBC patients enrolled in the ProfiLER-01 study to determine the prevalence of alterations in homologous recombination-related (HRR) genes and their association with platinum sensitivity.
Methods: next-generation sequencing and promoter methylation of BRCA1 and RAD51C were performed on tumors from patients with mTNBC, using a panel of 19 HRR genes. Tumors were separated into three groups based on their molecular status: mutations in BRCA1/2, mutations in other HRR genes (BRCA1/2 excluded) or BRCA1/RAD51C promoter methylation and the absence of molecular alterations in HRR genes (groups A, B and C, respectively). Sensitivity to platinum-based chemotherapy was evaluated through the radiological response.
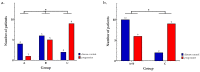
Results: mutations in BRCA1/2 were detected in seven (13.5%) patients, while alterations in other HRR genes or hypermethylation in BRCA1 or RAD51C were reported in 16 (30.7%) patients; furthermore, no alteration was found in the majority of patients (n = 29; 55.8%). Among 27 patients who received platinum-based chemotherapy, the disease control rate was 80%, 55% and 18% (groups A, B and C, respectively; p = 0.049). Regarding group B, patients with disease control exhibited mutations in FANCL, FANCA and the RAD51D genes or RAD51C methylation; Conclusion: mutations in HRR genes and epimutations in RAD51C were associated with disease control through platinum-based chemotherapy. As such, apart from well-characterized alterations in BRCA1/2, a more comprehensive evaluation of HRD should be considered in order to enlarge the selection of patients with mTNBC that could benefit from platinum-based chemotherapy.
Keywords: BRCA; DNA repair; HRR genes; TNBC; homologous recombination; platinum-based chemotherapy; triple-negative breast cancer.
Conflict of interest statement
J.-Y.B.: received research support and honoraria from Astrazeneca, GSK, Novartis; P.-E.H. received grants and non-financial support from AstraZeneca, Roche, Novartis, Pfizer, personal fees from Mylan, personal fees and non-financial support from EISAI, outside the submitted work; I.R.-C.: received honoraria from AstraZeneca, Clovis, Tesaro and PharmaMar; Consulting/advisory board fees from AstraZeneca, Roche, Clovis, Tesaro, Genmab, PharmaMar, MSD and Pfizer, research funding from MSD and BMS, travel expenses from AstraZeneca, GSK and Roche; O.T.: received grants from Roche, MSD-Merck, BMS; personal fees from Roche, MSD-Merck, Novartis-Sandoz, Pfizer, Lilly, Astra-Zeneca, Daiichi Sankyo, Eisai, Pierre Fabre; D.P. (David Pérol): received honoraria from Astrazeneca, BMS, ELI-Lilly, IPSEN, Roche, Novartis, Pierre Fabre, MSD and Takeda, research funding from MSD Avenir, travel expenses from Astrazeneca; T.B.: received grants, advisory board fees and non-financial support from Novartis, AstraZeneca and Pfizer, advisory board fees and non-financial support from Roche, advisory board fees from SeattleGenetics. E.B., V.H., S.Q., K.-A.B., A.L.-C., Q.W., L.E., L.C., V.B., I.T., V.A., V.C., C.L., A.D., E.S., D.P. (Daniel Pissaloux), A.V., S.P., A.B. and P.T. declare that they have no conflict of interest.
Figures


References
-
- Mahtani R., Kittaneh M., Kalinsky K., Mamounas E., Badve S., Vogel C., Lower E., Schwartzberg L., Pegram M., Breast Cancer Therapy Expert Group (BCTEG) Advances in Therapeutic Approaches for Triple-Negative Breast Cancer. Clin. Breast Cancer. 2021;21:383–390. doi: 10.1016/j.clbc.2020.12.011. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

