Olive Leaf Extract Attenuates Chlorpyrifos-Induced Neuro- and Reproductive Toxicity in Male Albino Rats
- PMID: 36294935
- PMCID: PMC9605092
- DOI: 10.3390/life12101500
Olive Leaf Extract Attenuates Chlorpyrifos-Induced Neuro- and Reproductive Toxicity in Male Albino Rats
Abstract
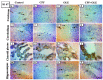
Chlorpyrifos (CPF) is a common organophosphorus insecticide. It is associated with negative consequences such as neurotoxicity and reproductive injury. This study aimed to observe the ability of olive leaf extract to attenuate chlorpyrifos toxicity, which induced neuro- and reproductive toxicity in male albino rats. Olive leaf extract (OLE) exhibits potent antioxidant and antiapoptotic properties. Twenty-two mature male rats were divided into four groups: control (saline), CPF (9 mg/kg), OLE (150 mg/kg), and CPF + OLE. Treatment was administered orally for 80 days. The CPF significantly reduced serum sex hormones, sperm counts and motility, high oxidants (MDA), and depleted antioxidants (GSH, SOD, TAC) in the brain and testes homogenate; additionally, it decreased serum AChE and brain neurotransmitters, increased Bax, decreased Bcl-2, and boosted caspase-3 immune expression in neural and testicular cells. Immunological expression of Ki 67 in the cerebrum, cerebellum, choroid plexus, and hippocampus was reduced, and α-SMA in testicular tissue also decreased. Histopathological findings were consistent with the above impacts. OLE co-administration significantly normalized all these abnormalities. OLE showed significant protection against neural and reproductive damage caused by CPF.
Keywords: apoptosis; chlorpyrifos; neurotoxicity; olive leaf extract; oxidative stress; reproductive toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Khalaf A., Ibrahim M., Tohamy A., Allah A., Zaki A.R. Protective effect of vitazinc on chlorsan induced oxidative stress, genotoxicity and histopathological changes in testicular tissues of male rats. Int. J. Pharmacol. 2017;13:22–32. doi: 10.3923/ijp.2017.22.32. - DOI
-
- Singh N., Lawana V., Luo J., Phong P., Abdalla A., Palanisamy B., Rokad D., Sarkar S., Jin H., Anantharam V. Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: Implications for mitochondria mediated oxidative stress signaling events. Neurobiol. Dis. 2018;117:82–113. doi: 10.1016/j.nbd.2018.05.019. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

