Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater
- PMID: 36294969
- PMCID: PMC9605590
- DOI: 10.3390/life12101534
Confocal Raman Micro-Spectroscopy for Discrimination of Glycerol Diffusivity in Ex Vivo Porcine Dura Mater
Abstract
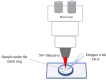
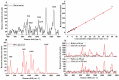
Dura mater (DM) is a connective tissue with dense collagen, which is a protective membrane surrounding the human brain. The optical clearing (OC) method was used to make DM more transparent, thereby allowing to increase in-depth investigation by confocal Raman micro-spectroscopy and estimate the diffusivity of 50% glycerol and water migration. Glycerol concentration was obtained, and the diffusion coefficient was calculated, which ranged from 9.6 × 10-6 to 3.0 × 10-5 cm2/s. Collagen-related Raman band intensities were significantly increased for all depths from 50 to 200 µm after treatment. In addition, the changes in water content during OC showed that 50% glycerol induces tissue dehydration. Weakly and strongly bound water types were found to be most concentrated, playing a major role in the glycerol-induced water flux and OC. Results show that OC is an efficient method for controlling the DM optical properties, thereby enhancing the in-depth probing for laser therapy and diagnostics of the brain. DM is a comparable to various collagen-containing tissues and organs, such as sclera of eyes and skin dermis.
Keywords: collagen type I; dehydration; diffusion coefficients; glycerol; high wavenumber; hydrogen bound water; penetration; topical application.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures




References
-
- Latka I., Dochow S., Krafft C., Dietzek B., Popp J. Fiber optic probes for linear and nonlinear Raman applications-Current trends and future development. Laser Photonics Rev. 2013;7:698–731. doi: 10.1002/lpor.201200049. - DOI
-
- Lee K.S., Landry Z., Pereira F.C., Wagner M., Berry D., Huang W.E., Taylor G.T., Kneipp J., Popp J., Zhang M., et al. Raman microspectroscopy for microbiology. Nat. Rev. Methods Prim. 2021;1:1–25. doi: 10.1038/s43586-021-00075-6. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

