Single Cell in a Gravity Field
- PMID: 36295035
- PMCID: PMC9604728
- DOI: 10.3390/life12101601
Single Cell in a Gravity Field
Abstract
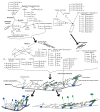
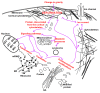
The exploration of deep space or other bodies of the solar system, associated with a long stay in microgravity or altered gravity, requires the development of fundamentally new methods of protecting the human body. Most of the negative changes in micro- or hypergravity occur at the cellular level; however, the mechanism of reception of the altered gravity and transduction of this signal, leading to the formation of an adaptive pattern of the cell, is still poorly understood. At the same time, most of the negative changes that occur in early embryos when the force of gravity changes almost disappear by the time the new organism is born. This review is devoted to the responses of early embryos and stem cells, as well as terminally differentiated germ cells, to changes in gravity. An attempt was made to generalize the data presented in the literature and propose a possible unified mechanism for the reception by a single cell of an increase and decrease in gravity based on various deformations of the cortical cytoskeleton.
Keywords: cellular mechanoreception; cellular mechanotransduction; early embryo; hypergravity; microgravity; oocyte; space flight; spermatozoon.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Oganov V.S. Modern analysis of bone loss mechanisms in microgravity. J. Gravit. Physiol. 2004;11:143–146. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

