Guanidine Derivatives of Quinazoline-2,4(1 H,3 H)-Dione as NHE-1 Inhibitors and Anti-Inflammatory Agents
- PMID: 36295082
- PMCID: PMC9605072
- DOI: 10.3390/life12101647
Guanidine Derivatives of Quinazoline-2,4(1 H,3 H)-Dione as NHE-1 Inhibitors and Anti-Inflammatory Agents
Abstract
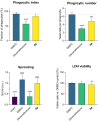
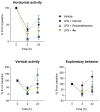
Quinazolines are a rich source of bioactive compounds. Previously, we showed NHE-1 inhibitory, anti-inflammatory, antiplatelet, intraocular pressure lowering, and antiglycating activity for a series of quinazoline-2,4(1H,3H)-diones and quinazoline-4(3H)-one guanidine derivatives. In the present work, novel N1,N3-bis-substituted quinazoline-2,4(1H,3H)-dione derivatives bearing two guanidine moieties were synthesized and pharmacologically profiled. The most potent NHE-1 inhibitor 3a also possesses antiplatelet and intraocular-pressure-reducing activity. Compound 4a inhibits NO synthesis and IL-6 secretion in murine macrophages without immunotoxicity and alleviates neutrophil infiltration, edema, and tissue lesions in a model of LPS-induced acute lung injury. Hence, we considered quinazoline derivative 4a as a potential agent for suppression of cytokine-mediated inflammatory response and acute lung injury.
Keywords: LPS; NHE-1; cytokine release; lung injury; macrophage; quinazoline-2,4(1H,3H)-dione.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; nor in the decision to publish the results.
Figures






References
-
- Haddad J.J., Land S.C. Amiloride Blockades Lipopolysaccharide-Induced Proinflammatory Cytokine Biosynthesis in an I κ B-α/NF-κ B–Dependent Mechanism: Evidence for the Amplification of an Antiinflammatory Pathway in the Alveolar Epithelium. Am. J. Respir. Cell Mol. Biol. 2002;26:114–126. doi: 10.1165/ajrcmb.26.1.4657. - DOI - PubMed
-
- Adapted from “NF-KB Signaling Pathway”, by BioRender.com. 2022. [(accessed on 3 October 2022)]. Available online: https://app.biorender.com/biorender-templates.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

