Predicting the Potentiometric Sensitivity of Membrane Sensors Based on Modified Diphenylphosphoryl Acetamide Ionophores with QSPR Modeling
- PMID: 36295713
- PMCID: PMC9611910
- DOI: 10.3390/membranes12100953
Predicting the Potentiometric Sensitivity of Membrane Sensors Based on Modified Diphenylphosphoryl Acetamide Ionophores with QSPR Modeling
Abstract
While potentiometric, plasticized membrane sensors are known as convenient, portable and inexpensive analytical instruments, their development is time- and resource-consuming, with a poorly predictable outcome. In this study, we investigated the applicability of the QSPR (quantitative structure-property relationship) method for predicting the potentiometric sensitivity of plasticized polymeric membrane sensors, using the ionophore chemical structure as model input. The QSPR model was based on the literature data on sensitivity, from previously studied, structurally similar ionophores, and it has shown reasonably good metrics in relating ionophore structures to their sensitivities towards Cu2+, Cd2+ and Pb2+. The model predictions for four newly synthesized diphenylphosphoryl acetamide ionophores were compared with real potentiometric experimental data for these ionophores, and satisfactory agreement was observed, implying the validity of the proposed approach.
Keywords: QSPR; heavy metals sensing; ion-selective electrodes; potentiometric sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
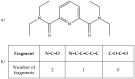

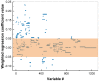

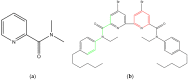
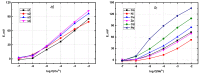
References
-
- Forster R.J., Keyes T.E. Ion-selective Electrodes in Environmental Analysis. In: Meyers R.A., Miller M.P., editors. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2006. - DOI
-
- Maj-Żurawska M., Hulanicki A. ION-SELECTIVE ELECTRODES|Food Applications. In: Worsfold P., Poole C., Townshend A., Miró M., editors. Encyclopedia of Analytical Science. 3rd ed. Academic Press; Cambridge, MA, USA: 2013. pp. 226–230. - DOI
-
- Bobacka J. Conducting Polymer-Based Solid-State Ion-Selective Electrodes. Electroanalysis. 2006;18:7–18. doi: 10.1002/elan.200503384. - DOI
LinkOut - more resources
Full Text Sources

