Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer
- PMID: 36296073
- PMCID: PMC9607611
- DOI: 10.3390/mi13101720
Design and High-Resolution Analysis of an Efficient Periodic Split-and-Recombination Microfluidic Mixer
Abstract
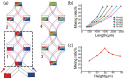
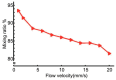
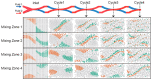
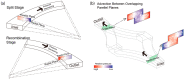
We developed a highly efficient passive mixing device based on a split-and-recombine (SAR) configuration. This micromixer was constructed by simply bonding two identical microfluidic periodical open-trench patterns face to face. The structure parameters of periodical units were optimized through numerical simulation to facilitate the mixing efficiency. Despite the simplicity in design and fabrication, it provided rapid mixing performance in both experiment and simulation conditions. To better illustrate the mixing mechanism, we developed a novel scheme to achieve high-resolution confocal imaging of serial channel cross-sections to accurately characterize the mixing details and performance after each SAR cycle. Using fluorescent IgG as an indicator, nearly complete mixing was achieved using only four SAR cycles in an aqueous solution within a device's length of less than 10 mm for fluids with a Péclet number up to 8.7 × 104. Trajectory analysis revealed that each SAR cycle transforms the input fluids using three synergetic effects: rotation, combination, and stretching to increase the interfaces exponentially. Furthermore, we identified that the pressure gradients in the parallel plane of the curved channel induced vertical convection, which is believed to be the driving force underlying these effects to accelerate the mixing process.
Keywords: lab-on-chip; microfabrication; microfluidic mixer; uTAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Mixing Performance of a Cost-effective Split-and-Recombine 3D Micromixer Fabricated by Xurographic Method.Micromachines (Basel). 2019 Nov 16;10(11):786. doi: 10.3390/mi10110786. Micromachines (Basel). 2019. PMID: 31744080 Free PMC article.
-
A "twisted" microfluidic mixer suitable for a wide range of flow rate applications.Biomicrofluidics. 2016 Jun 27;10(3):034120. doi: 10.1063/1.4954812. eCollection 2016 May. Biomicrofluidics. 2016. PMID: 27453767 Free PMC article.
-
Optimized design of obstacle sequences for microfluidic mixing in an inertial regime.Lab Chip. 2021 Oct 12;21(20):3910-3923. doi: 10.1039/d1lc00483b. Lab Chip. 2021. PMID: 34636817
-
Mixing Performance of a Cross-Channel Split-and-Recombine Micro-Mixer Combined with Mixing Cell.Micromachines (Basel). 2020 Jul 15;11(7):685. doi: 10.3390/mi11070685. Micromachines (Basel). 2020. PMID: 32679732 Free PMC article.
-
Modeling and simulation of a split and recombination-based passive micromixer with vortex-generating mixing units.Heliyon. 2023 Mar 24;9(4):e14745. doi: 10.1016/j.heliyon.2023.e14745. eCollection 2023 Apr. Heliyon. 2023. PMID: 37025873 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

