3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization
- PMID: 36296078
- PMCID: PMC9607065
- DOI: 10.3390/mi13101726
3D Scaffolds Fabrication via Bicomponent Microgels Assembly: Process Optimization and In Vitro Characterization
Abstract
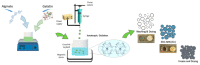
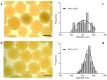
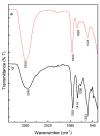
In the last decade, different technological approaches have been proposed for the fabrication of 3D models suitable to evaluate in vitro cell response. Among them, electro fluid dynamic atomization (EFDA) belonging to the family of electro-assisted technologies allows for the dropping of polysaccharides and/or proteins solutions to produce micro-scaled hydrogels or microgels with the peculiar features of hydrogel-like materials (i.e., biocompatibility, wettability, swelling). In this work, a method to fabricate 3D scaffolds by the assembly of bicomponent microgels made of sodium alginate and gelatin was proposed. As first step, optical and scanning electron microscopy with the support of image analysis enabled to explore the basic properties of single blocks in terms of correlation between particle morphology and process parameters (i.e., voltage, flow rate, electrode gap, and needle diameter). Chemical analysis via ninhydrin essays and FTIR analysis confirmed the presence of gelatin, mostly retained by physical interactions into the alginate network mediated by electrostatic forces. In vitro tests confirmed the effect of biochemical signals exerted by the protein on the biological response of hMSCs cultured onto the microgels surface. Hence, it is concluded that alginate/gelatin microgels assemblies can efficiently work as 3D scaffolds able to support in vitro cells functions, thus providing a friendly microenvironment to investigate in vitro cell interactions.
Keywords: alginate; biocompatibility; gelatin; hMSC; scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Polydopamine-Coated Alginate Microgels: Process Optimization and In Vitro Validation.J Funct Biomater. 2022 Dec 20;14(1):2. doi: 10.3390/jfb14010002. J Funct Biomater. 2022. PMID: 36662049 Free PMC article.
-
3D Printed Chitosan Composite Scaffold for Chondrocytes Differentiation.Curr Med Imaging. 2021;17(7):832-842. doi: 10.2174/1573405616666201217112939. Curr Med Imaging. 2021. PMID: 33334294
-
Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study.Mater Sci Eng C Mater Biol Appl. 2020 Aug;113:110957. doi: 10.1016/j.msec.2020.110957. Epub 2020 Apr 12. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32487379
-
Hydrogels and microtechnologies for engineering the cellular microenvironment.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012 May-Jun;4(3):235-46. doi: 10.1002/wnan.171. Epub 2011 Dec 5. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012. PMID: 22144036 Review.
-
Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs.Expert Rev Med Devices. 2016;13(1):83-102. doi: 10.1586/17434440.2016.1126505. Epub 2015 Dec 19. Expert Rev Med Devices. 2016. PMID: 26619260 Review.
Cited by
-
Designing Advanced Drug Delivery Systems: Core-Shell Alginate Particles through Electro-Fluid Dynamic Atomization.Pharmaceutics. 2024 Jan 29;16(2):193. doi: 10.3390/pharmaceutics16020193. Pharmaceutics. 2024. PMID: 38399251 Free PMC article.
-
Polydopamine-Coated Alginate Microgels: Process Optimization and In Vitro Validation.J Funct Biomater. 2022 Dec 20;14(1):2. doi: 10.3390/jfb14010002. J Funct Biomater. 2022. PMID: 36662049 Free PMC article.
References
-
- Karg M., Pich A., Hellweg T., Hoare T., Lyon L.A., Crassous J.J., Suzuki D., Gumerov R.A., Schneider S., Potemkin I.I., et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir. 2019;35:6231–6255. doi: 10.1021/acs.langmuir.8b04304. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

