Paracoccidioides lutzii Formamidase Contributes to Fungal Survival in Macrophages
- PMID: 36296287
- PMCID: PMC9608497
- DOI: 10.3390/microorganisms10102011
Paracoccidioides lutzii Formamidase Contributes to Fungal Survival in Macrophages
Abstract
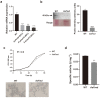
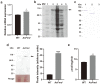
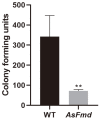
Nitrogen is a crucial nutrient for microorganisms that compose essential biomolecules. However, hosts limit this nutrient as a strategy to counter infections, therefore, pathogens use adaptive mechanisms to uptake nitrogen from alternative sources. In fungi, nitrogen catabolite repression (NCR) activates transcription factors to acquire nitrogen from alternative sources when preferential sources are absent. Formamidase has been related to nitrogen depletion in Aspergillus nidulans through formamide degradation to use the released ammonia as a nitrogen source. In Paracoccidioides spp., formamidase is highly expressed in transcriptomic and proteomic analyses. Here, we aim to investigate the importance of formamidase to Paracoccidioides lutzii. Thereby, we developed a P. lutzii silenced strain of fmd gene (AsFmd) by antisense RNA technology using Agrobacterium tumefaciens-mediated transformation (ATMT). The AsFmd strain led to increased urease expression, an enzyme related to nitrogen assimilation in other fungi, suggesting that P. lutzii might explore urease as an alternative route for ammonia metabolism as a nitrogen source. Moreover, formamidase was important for fungal survival inside macrophages, as fungal recovery after macrophage infection was lower in AsFmd compared to wild-type (WT) strain. Our findings suggest potential alternatives of nitrogen acquisition regulation in P. lutzii, evidencing formamidase influence in fungal virulence.
Keywords: gene silencing; macrophage infection; nitrogen catabolite repression; nitrogen depletion; virulence.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Shikanai-Yasuda M.A., Mendes R.P., Colombo A.L., de Queiroz-Telles F., Kono A.S.G., Paniago A.M.M., Nathan A., do Valle A.C.F., Bagagli E., Benard G., et al. Brazilian Guidelines for the Clinical Management of Paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017;50:715–740. doi: 10.1590/0037-8682-0230-2017. - DOI - PubMed
Grants and funding
- 408042/2021-4/National Council for Scientific and Technological Development
- INCT-IPH/Instituto Nacional de Ciência e Tecnologia
- 03/2016-PPSUS-MS, PPSUS/FUNDECT No. 08/2020/FUNDECT/DECIT-MS/CNPq/SES
- DS/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- 88882.458447/2019-01/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources
Miscellaneous

