Molecular Profiles of Multiple Antimalarial Drug Resistance Markers in Plasmodium falciparum and Plasmodium vivax in the Mandalay Region, Myanmar
- PMID: 36296297
- PMCID: PMC9612053
- DOI: 10.3390/microorganisms10102021
Molecular Profiles of Multiple Antimalarial Drug Resistance Markers in Plasmodium falciparum and Plasmodium vivax in the Mandalay Region, Myanmar
Abstract
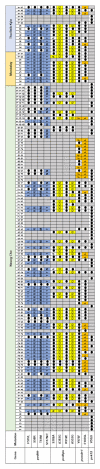
Emergence and spreading of antimalarial drug resistant malaria parasites are great hurdles to combating malaria. Although approaches to investigate antimalarial drug resistance status in Myanmar malaria parasites have been made, more expanded studies are necessary to understand the nationwide aspect of antimalarial drug resistance. In the present study, molecular epidemiological analysis for antimalarial drug resistance genes in Plasmodium falciparum and P. vivax from the Mandalay region of Myanmar was performed. Blood samples were collected from patients infected with P. falciparum and P. vivax in four townships around the Mandalay region, Myanmar in 2015. Partial regions flanking major mutations in 11 antimalarial drug resistance genes, including seven genes (pfdhfr, pfdhps, pfmdr-1, pfcrt, pfk13, pfubp-1, and pfcytb) of P. falciparum and four genes (pvdhfr, pvdhps, pvmdr-1, and pvk12) of P. vivax were amplified, sequenced, and overall mutation patterns in these genes were analyzed. Substantial levels of mutations conferring antimalarial drug resistance were detected in both P. falciparum and P. vivax isolated in Mandalay region of Myanmar. Mutations associated with sulfadoxine-pyrimethamine resistance were found in pfdhfr, pfdhps, pvdhfr, and pvdhps of Myanmar P. falciparum and P. vivax with very high frequencies up to 90%. High or moderate levels of mutations were detected in genes such as pfmdr-1, pfcrt, and pvmdr-1 associated with chloroquine resistance. Meanwhile, low frequency mutations or none were found in pfk13, pfubp-1, pfcytb, and pvk12 of the parasites. Overall molecular profiles for antimalarial drug resistance genes in malaria parasites in the Mandalay region suggest that parasite populations in the region have substantial levels of mutations conferring antimalarial drug resistance. Continuous monitoring of mutations linked with antimalarial drug resistance is necessary to provide useful information for policymakers to plan for proper antimalarial drug regimens to control and eliminate malaria in the country.
Keywords: Myanmar; Plasmodium falciparum; Plasmodium vivax; drug resistance genes; malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization . World Malaria Report. WHO; Geneva, Switzerland: 2021.
Grants and funding
LinkOut - more resources
Full Text Sources

