One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance
- PMID: 36296408
- PMCID: PMC9610260
- DOI: 10.3390/molecules27206816
One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance
Abstract
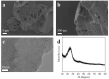
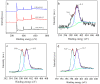
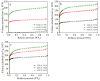
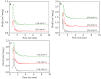
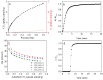
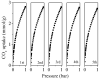
N-enriched porous carbons have played an important part in CO2 adsorption application thanks to their abundant porosity, high stability and tailorable surface properties while still suffering from a non-efficient and high-cost synthesis method. Herein, a series of N-doped porous carbons were prepared by a facile one-pot KOH activating strategy from commercial urea formaldehyde resin (UF). The textural properties and nitrogen content of the N-doped carbons were carefully controlled by the activating temperature and KOH/UF mass ratios. As-prepared N-doped carbons show 3D block-shaped morphology, the BET surface area of up to 980 m2/g together with a pore volume of 0.52 cm3/g and N content of 23.51 wt%. The optimal adsorbent (UFK-600-0.2) presents a high CO2 uptake capacity of 4.03 mmol/g at 0 °C and 1 bar. Moreover, as-prepared N-doped carbon adsorbents show moderate isosteric heat of adsorption (43-53 kJ/mol), acceptable ideal adsorption solution theory (IAST) selectivity of 35 and outstanding recycling performance. It has been pointed out that while the CO2 uptake was mostly dependent on the textural feature, the N content of carbon also plays a critical role to define the CO2 adsorption performance. The present study delivers favorable N-doped carbon for CO2 uptake and provides a promising strategy for the design and synthesis of the carbon adsorbents.
Keywords: CO2 adsorption; N-doped; one-pot KOH activation; porous carbon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yu C.-H., Huang C.-H., Tan C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol. Air Qual. Res. 2012;12:745–769. doi: 10.4209/aaqr.2012.05.0132. - DOI
-
- Feron P.H., Cousins A., Jiang K., Zhai R., Garcia M. An update of the benchmark post-combustion CO2-capture technology. Fuel. 2020;273:117776. doi: 10.1016/j.fuel.2020.117776. - DOI
-
- Yan H.Y., Zhang G.J., Xu Y., Zhang Q.Q., Liu J., Li G.Q., Zhao Y.Q., Wang Y., Zhang Y.F. High CO2 adsorption on amine-functionalized improved macro-/mesoporous multimodal pore silica. Fuel. 2022;315:123195. doi: 10.1016/j.fuel.2022.123195. - DOI
-
- Shen S., Shi X., Li C., Guo H., Long Q., Wang S., Yin X. Nonaqueous (amine + glycol ether) solvents for energy-efficient CO2 capture: New insights into phase change behaviors and assessment of capture performance. Sep. Purif. Technol. 2022;300:121908. doi: 10.1016/j.seppur.2022.121908. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

