Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3
- PMID: 36296482
- PMCID: PMC9607513
- DOI: 10.3390/molecules27206889
Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3
Abstract
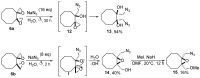
Reactions of oxirane ring opening provide a powerful tool for regio- and stereoselective synthesis of polyfunctional and heterocyclic compounds, widely used in organic chemistry and drug design. Cyclooctane, alongside other medium-sized rings, is of interest as a novel molecular platform for the construction of target-oriented leads. Additionally, cyclooctane derivatives are well known to be prone to transannular reactions, which makes them a promising object in the search for novel approaches to polycyclic structures. In the present work, a series of cyclooctanediones was studied in Corey-Chaykovsky reactions, and novel spirocyclic bis(oxiranes) containing cyclooctane core, namely, 1,5-dioxadispiro[2.0.2.6]dodecane and 1,8-dioxadispiro[2.3.2.3]dodecane, were synthesized. Ring opening of the obtained bis(oxiranes) upon treatment with sodium azide was investigated, and it was found that the reaction path is determined by the reciprocal orientation of oxygen atoms in the oxirane moieties. Diastereomers of the bis(oxiranes) with cis-orientation underwent independent ring opening, supplying corresponding diazidodiols, while in the case of stereoisomers with trans-orientation, domino-like reactions occurred, including intramolecular nucleophilic attack and the formation of a novel three- or six-membered O-containing ring. Summarily, a straightforward approach to polyfunctional compounds containing cyclooctane or oxabicyclo[3.3.1]nonane cores, employing bis(oxiranes), was elaborated.
Keywords: azides; cyclooctanes; domino reactions; nucleophilic ring opening; oxabicyclo[3.3.1]nonanes; oxiranes; polyfunctional compounds; polyols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

