Hydroxy-3-Phenylcoumarins as Multitarget Compounds for Skin Aging Diseases: Synthesis, Molecular Docking and Tyrosinase, Elastase, Collagenase and Hyaluronidase Inhibition, and Sun Protection Factor
- PMID: 36296507
- PMCID: PMC9611449
- DOI: 10.3390/molecules27206914
Hydroxy-3-Phenylcoumarins as Multitarget Compounds for Skin Aging Diseases: Synthesis, Molecular Docking and Tyrosinase, Elastase, Collagenase and Hyaluronidase Inhibition, and Sun Protection Factor
Abstract
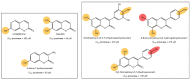
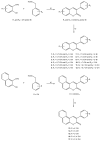
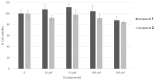
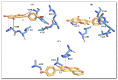
Skin aging is a progressive biological process of the human body, and it is not only time-dependent. Differently substituted 3-phenylcoumarins proved to efficiently inhibit tyrosinase. In the current work, new substitution patterns have been explored, and the biological studies were extended to other important enzymes involved in the processes of skin aging, as elastase, collagenase and hyaluronidase. From the studied series, five compounds presented inhibitory activity against tyrosinase, one compound against elastase, eight compounds against collagenase and two compounds against hyaluronidase, being five compounds dual inhibitors. The 3-(4'-Bromophenyl)-5,7-dihydroxycoumarin (1) and 3-(3'-bromophenyl)-5,7-dihydroxycoumarin (2) presented the best profiles against tyrosinase (IC50 = 1.05 µM and 7.03 µM) and collagenase (IC50 = 123.4 µM and 110.4 µM); the 3-(4'-bromophenyl)-6,7-dihydroxycoumarin (4) presented a good inhibition against tyrosinase and hyaluronidase; the 3-(3'-bromophenyl)-6,7-dihydroxycoumarin (5) showed an effective tyrosinase and elastase inhibition; and 6,7-dihydroxy-3-(3'-hydroxyphenyl)coumarin (11) presented a dual profile inhibition against collagenase and hyaluronidase. Furthermore, considering the overall activities tested, compounds 1 and 2 proved to be the most promising anti-aging compounds. These compounds also showed to have a photo-protective effect, without being cytotoxic to human skin keratinocyte cells. To predict the binding site with the target enzymes, computational studies were also carried out.
Keywords: collagenase; elastase; hyaluronidase; hydroxy-3-phenylcoumarins; molecular docking; skin aging; sun protection factor; tyrosinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging.Bioorg Chem. 2018 Apr;77:159-167. doi: 10.1016/j.bioorg.2017.12.030. Epub 2018 Jan 4. Bioorg Chem. 2018. PMID: 29353733
-
Identification of chia seed (Salvia hispanica L.) peptides with enzyme inhibition activity towards skin-aging enzymes.Amino Acids. 2020 Aug;52(8):1149-1159. doi: 10.1007/s00726-020-02879-4. Epub 2020 Jul 30. Amino Acids. 2020. PMID: 32734432
-
Xanthones for melanogenesis inhibition: Molecular docking and QSAR studies to understand their anti-tyrosinase activity.Bioorg Med Chem. 2021 Jan 1;29:115873. doi: 10.1016/j.bmc.2020.115873. Epub 2020 Nov 19. Bioorg Med Chem. 2021. PMID: 33242700
-
Use of chromatographic and electrophoretic tools for assaying elastase, collagenase, hyaluronidase, and tyrosinase activity.J Chromatogr A. 2017 Dec 22;1529:1-28. doi: 10.1016/j.chroma.2017.11.003. Epub 2017 Nov 9. J Chromatogr A. 2017. PMID: 29132826 Review.
-
Cyanobacteria and microalgae bioactive compounds in skin-ageing: potential to restore extracellular matrix filling and overcome hyperpigmentation.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1829-1838. doi: 10.1080/14756366.2021.1960830. J Enzyme Inhib Med Chem. 2021. PMID: 34353202 Free PMC article. Review.
Cited by
-
Coumarin-Based Compounds as Inhibitors of Tyrosinase/Tyrosine Hydroxylase: Synthesis, Kinetic Studies, and In Silico Approaches.Int J Mol Sci. 2023 Mar 9;24(6):5216. doi: 10.3390/ijms24065216. Int J Mol Sci. 2023. PMID: 36982292 Free PMC article.
-
Design, Synthesis, Structural Insights, Tyrosinase Inhibition, and Sun Protection Factor of New Thiosemicarbazone Derivatives.Molecules. 2024 Nov 28;29(23):5629. doi: 10.3390/molecules29235629. Molecules. 2024. PMID: 39683787 Free PMC article.
-
Potential of Bioactive Protein and Protein Hydrolysate from Apis mellifera Larvae as Cosmeceutical Active Ingredients for Anti-Skin Aging.Pharmaceuticals (Basel). 2024 May 24;17(6):679. doi: 10.3390/ph17060679. Pharmaceuticals (Basel). 2024. PMID: 38931346 Free PMC article.
-
Heterocyclic Compounds as Synthetic Tyrosinase Inhibitors: Recent Advances.Int J Mol Sci. 2023 May 22;24(10):9097. doi: 10.3390/ijms24109097. Int J Mol Sci. 2023. PMID: 37240442 Free PMC article. Review.
-
Essential Oil Compounds of Andaliman (Zanthoxylum acanthopodium DC.) Fruit Varieties and Their Utilization as Skin Anti-Aging Using Molecular Docking.Life (Basel). 2023 Mar 10;13(3):754. doi: 10.3390/life13030754. Life (Basel). 2023. PMID: 36983909 Free PMC article.
References
-
- Rosa G.P., Palmeira A., Resende D.I.S.P., Almeida I.F., Kane-Pagès A., Barreto M.C., Sousa E., Pinto M.M.M. Xanthones for melanogenesis inhibition: Molecular docking and QSAR studies to understand their anti-tyrosinase activity. Biorg. Med. Chem. 2021;29:115873. doi: 10.1016/j.bmc.2020.115873. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

